Seema Rohilla4, Sanjeev Parsad3
1 Professor; 2 Medical officer
Department of Anesthesiology, Pt BDS PGIMS Rohtak, Haryana, (India)
3 Professor of Surgery, Pt BDS PGIMS Rohtak, Haryana, (India)
4 Professor of Radiology, Pt BDS PGIMS Rohtak, Haryana, (India)
Correspondence:Dr Anju Ghai, 19/9 J Medical Campus, Pt B D Sharma PGIMS Rohtak, Haryana (India); Phone: 01262-212860, 919416974794; E-mail: dr.wadhera@yahoo.com
ABSTRACT
Objectives: Celiac plexus neurolysisisadjunct modalityto relieve intractable pain caused by upper abdominal malignancy. An anterior approach offers advantages including shorter procedure time, less discomfort and less risk of neurological complications. TheCT and ultrasound helpto improve visualization of the celiac plexus. Their use allow accurate needle placement and reduce the risks. We report our experience with sonographically guided anterior approach to celiac plexus neurolysisin upper abdominal malignancy patients.
Methodology: Patients with upper abdominal malignancy with VAS≥3 not responding to diclofenac and demanding additional opioidsor those having adverse effects were included. A prognosticblock was performed under deep sedation withsonographic guidance using 22G, 15cm long Chiba needle advanced through biopsy guide to thepreaortic zone above takeoff of celiac artery. Thirty to forty ml of 50% alcohol was injected. The VAS scores, analgesic consumption,duration of complete and partial pain reliefwere assessed at one hour, 24 hours, one week, one month, two month and three month intervals.
Results: Fifteen patients were enrolled There was statistically significant decrease in mean VAS score at 1st hour, 24th hour, 1st week, 1st, 2nd and 3rd month respectively (p< 0.05). The analgesic consumption was statistically significant at all time intervals from baseline (p< 0.05).
Conclusion: Use ofcolordopplerhelps in real time positioning of needleIt issuccessful in terminally ill patients.
Key words: Celiac plexus;Abdominal neoplasms; Abdominal pain;Abdominal Pain/etiology; Chronic pain; Pain Measurement; Pancreatitis/complications; Autonomic Nerve Block/methods; Bupivacaine; Endosonography; Humans; Pain, Intractable; Ultrasonography
Citation: Ghai A, Kumar H, Karwasra RK, Kad N, Rohilla S, Parsad S. Ultrasound guided celiac plexus neurolysis by anterior approach for pain management in upper abdominal malignancy: Our experience. Anaesth Pain & Intensive Care 2015;19(3):274-281
INTRODUCTION
Malignant tumors originating from pancreas, stomach, liver, gallbladder and lymph nodes or chronic pancreatitis may cause abdominal pain, which is unresponsive to large doses of narcotic analgesics, andimpairpatient’s quality of life. The symptoms of disease appear usually in advanced stagesafter considerable tumor growth and metastatic spread. The majority of these cases are non-resectable and highly resistant to conventional chemoradiation therapy leading to poor prognosis. Narcotic analgesics serve asmainstay of pain management. However due to severity of pain, opioids are effective only in dosages that induce significant side effects such as constipation, nausea, vomiting, anorexia, drowsiness, delirium and addiction.
Coeliac plexus block(CPB)is an adjunct modality forpalliative care for abdominal malignancy patients.1It involves the injection of a neurolytic agent (most commonly absolute alcohol) into or around the celiac plexus to disrupt these neural impulses and control pain. Since Kappis2 described the percutaneous neurolytic celiac plexus block, improvements in this technique have been proposed. Several techniques have been proposed in an attempt to increase success rate, reduce morbidity and enhance technical accuracy of the block.3 The routes used are anterior transabdominal and posterior transcrural under guidance from fluoroscopy, computerized tomography4-7 or at the time of laparotomy by isolating the celiac artery.8Every procedure cannot be performed in CT room6 and needle tip cannot be precisely placed using bony landmarks due tovariations in soft tissue anatomy. Recent advances in ultrasonography have made it an attractive technology. This technique is cheaper and faster than computerized tomography guided method.4,9It alsoavoids complications associated with posterior approach. Advantages include comfortable position, single puncture, no risk of paralysis and real time Doppler to visualize vessels. Few authors have used sonographic guided anterior approach for CPN,10,11 so we hypothesized that sonographic neurolysis will be effective in patients with upper abdominal malignancy in whom systemic analgesics were ineffective.
Methodology
After obtaining institutional approval from the hospital ethical committee and informed consent, patients with upper abdominal malignancy with severe abdominal pain from May 2011 to Dec 2012 were included in the study at Pt BDS, PGIMS, Rohtak. Inclusion criterion included pain score three or more with drug therapy according to visual analogue scale or appearance of adverse effects to pharmacotherapy. Patients with coagulopathy, sepsis at site, intra-abdominal sepsis or bowel obstruction were excluded. Patients on anticoagulants or antiplatelet agents were asked to stop their medication to allow normalization of hemostasis
At admission following data were evaluated; beginning of early symptoms of disease (e.g. nausea, vomiting, weight loss, and jaundice), beginning and evaluation of pain symptoms and pain characteristics. Severity of pain was assessed on a 10 cm visual analogue scale (VAS) where zero represented no pain and 10 cm represented worst possible pain.Use of narcotic and non-narcotic analgesics and other medications- tranquilizers, antidepressants andhypnotics were noted. Patients wereasked to rate pain. Ultrasonography/computed tomography scan/magnetic resonance image findings of the abdomen were noted. The procedure was performed under deep sedation with intravenous fentanyl/ tramadol.Patient was kept fasting for 8 hours. Gut was prepared with four tablets of bisacodyl 5mg and six charcoal tablets the night before procedure. Patients were premeditated with tablet midazolam 15 mg and morphine 20 mg one hour prior to block.Anti-hypertensive medication was continued to prevent the risk of rebound hypertension. Prophylactic antibiotic was administered with injection amoxicillin 1 gm and clavulanic acid 200 mg one hour prior. All patients received intravenous fluids in the form of lactated ringer’s solution 10-15 ml/kg. Patient’s heart rate, blood pressure and oxygen saturation were monitored during the procedure.
The block was performed in the supine position.Ultrasonography was done by ACUSON S2000™ ultrasound system(Siemens) with high frequency curvilinear probe (3-5 MHz) with biopsy transducer attached. After cleaning and transducer preparation abdominal aorta and celiac trunk were localized by the radiologist by following descending aorta from the distal esophagus to the point where the celiac artery takeoff was identified. Lidocaine 1% was infiltrated at this point of entry. A 22G Chiba needle was introduced through biopsy guide transgastrically under guidance and advanced to reach the preaortic zone at the celiac trunk level, identified by colored doppler imaging. To improve ultrasound visualization of the fine needle, a colored doppler ultrasonography (CDU) detectable flow at the tip of 22G needle through continuous injection of 10 to 15 ml sterile saline was used. This technique revealed the exact position of the needle dynamically during its progression as well as its location in relation to the celiac trunk. Once the needle was in the anterocephalad position to the celiac artery takeoff, three ml of sterile normal saline was injected to flush the needle, which was followed by ten to fifteen ml of 1% lidocaine. The spread of solution was localized around the aorta at the level of the celiac trunk, predominantly in the preaortic zone. If the injection successfully relieved pain on the table, neurolysis with 30 to 40 ml of 50% alcohol was carried out. An echogenic cloud seen at the target site after alcohol injection confirmed that the substance was injected in the region of the celiac artery takeoff. The needle track was cleared with normal saline during withdrawal of the needle to avoid tracking of the neurolytic solution along the needle path.
Time to administer the block was noted, defined as from the entry of needle in the skin to localization of celiac plexus. Vital signs were noted in the first hour after the block. Patient was kept under close observation in the ward. Hydration was maintained with intravenous balanced salt solution to prevent hypotension. Elastic stockings and the use of pillows under the legs were advised. The compensatory vasomotor reflexes appeared within 24 to 36 hours, and patients were allowed ambulation as their postural symptoms allowed. Patients with VAS ≥ 3 were given analgesics in the form of combination of tramadol 37.5 mg and paracetamol 375 mg tablet three times a day. If VAS score remained more than three then patients were switched over to tablet morphine 5mg three times a day. Pain intensity for VAS equal to or less than three was criteria for effective pain relief after CPN. Patients were assessed for pain relief after one hour and twenty four hours after the block. Any complications in the form of local pain, postural hypotension, diarrhea were noted. Further follow up at one week, one month, two month and three month was made by telephonic contact with the patient. When necessary an outpatient clinic appointment was arranged to ensure the accuracy of received data and to determine and assess the patient condition. Quality of block was graded excellent, good, satisfactory or unsatisfactory according to patient’s assessment regarding pain relief. In addition analgesic requirement after CPN was monitored and noted. Pain scores and analgesic consumption were compared over time. Quality of block, duration of pain relief was noted. Duration of complete pain relief was up to time when VAS score was zero without analgesics after block. Duration of partial pain relief was taken as when VAS score was less than preblock period with less or same analgesic consumption.
Statistical analysis
Nonparametric methods were used. All the parameters were compiled and statistical analysis was done using SPSS for windows version 15.0 software. The variables are expressed as mean (SD) along with rang. The pain scores and analgesic usage pre- and post celiac plexus block were compared using the Wilcoxon signed rank test. A positive response was defined as a decrease in pain score ≥ 3. A p value of < 0.05 was considered statistically significant.
RESULTS
A total of 17 blocks were performed. Fifteen consecutive patients (6 males and 9 females) with a mean age of 54.67 ± 14.88 years (range 16-75 years) were enrolled in this prospective study. All patients had documented upper abdominal malignancy based on the findings of computed tomography scan or magnetic resonant image scan or ultrasonographic examination. All the patients presented with initial symptoms of nausea, vomiting, weight loss and anorexia while only four patients had jaundice. All of them complained of non-radiating deep and constant epigastric pain (Table 1). All enrolled subjects had failed response to narcotic analgesics. The mean preblock VAS was 8.1 ± 1 cm (Range 7-10 cm) and decreased to 0.53 ± 0.99 cm and 1 ± 2.22 cm at first week and first month respectively. It decreased further to 0.70 ± 1.64 cm and 0.33 ± 0.71 cm at 2nd month and 3rd month respectively. Figure 1 depicts the VAS at baseline and at one week for all the patients.
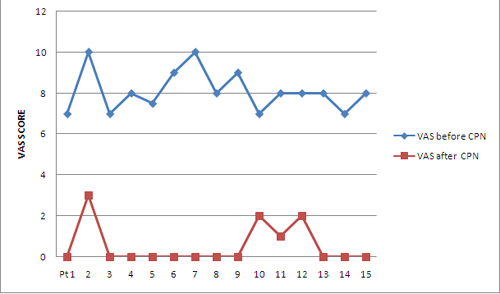
Figure 1: Comparison of VAS before and after CPN at one week,
There was statistically significant decrease in mean pre-block VAS score at 1st hour, 24th hour, 1st week, 1st, 2nd and 3rd month respectively (p< 0.05)(Table 2 and Figure 2).
Table 1: Patient characteristics, cancer disease and therapy
|
Parameter |
Value |
| Mean Age (years) |
54.67 ± 14.88 (Range 16-75 years) |
| Gender Male Female |
6 (40%) |
| Cancer disease Pancreas Gall bladder Ca rectum with liver metastasis |
11 (73.3%) |
| No. of patients With metastasis Without metastasis |
12 |
| Site of pain Epigastric Hypochondrial&Backpain |
15 patients |
| Accompanying symptoms Nausea, Vomiting, Weight loss Jaundice |
15 patients |
| Time from first symptom to CPN |
8.73±5.31 months |
| Time from diagnosis to CPN |
6.13±4.44 months |
| Previous therapies Surgical exploration Radiotherapy Chemotherapy |
9 |
|
Variable |
Baseline |
1st hour |
24th hour |
1st week |
1st month |
2nd month |
3rd month |
| VAS (cm) |
8.1 ± 1 |
0.87 ± 1.88* |
0.8 ± 1.7* |
0.53 ± 0.99* |
1 ± 2.22* |
0.70 ± 1.64* |
0.33 ± 0.71* |
| Range |
7-10 |
0-7 |
0-6 |
0-3 |
0-7 |
0-5 |
0-2 |
| p-value |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
All patients had a decrease in VAS score more than three from baseline at 1st hour, 24th hour, 1st week except one patient whose pain score fell from 7 to 6 and 2 at 24th hour and 1st week respectively. The decreased pain scores persisted in rest of eleven patients and one patient had an increase in VAS from zero at 1st week to 7 at 1st month, thereby explaining the increase in mean VAS at 1st month compared to baseline. One patient had a rise of pain score from zero at 1st month to 5 at 2nd month. Mean VAS at 2nd month decreased again as the rise in VAS of this patient was lesser (5 as compared to 7 for the patient at 1st month). Mean value of VAS at 3rd month again decreased further due to repeat blocks.
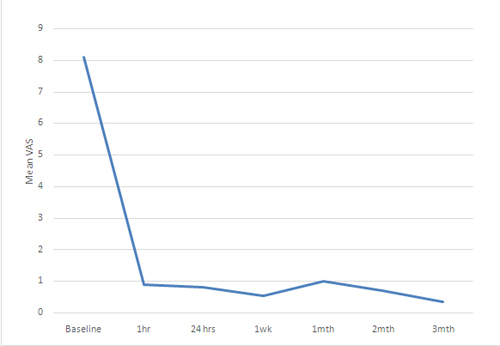
Figure 2: Mean visual analogue scale (VAS) scores in patients before and after neurolytic block at different time intervals
Mean time to localize celiac trunk was 7.73±2.71 min (Range 3-12 min). Depth of celiac trunk from skin was noted only in six patients. Mean depth was 7.38 cm (Range 4.6-13.8 cm).
Fourteen patients (93%) were taking a combination of tablet tramadol 37.5 mg and paracetamol 375 mg thrice daily. Out of these 7 patients (53%) were also taking tablet diclofenac 50 mg twice daily and one patient thrice daily, and 2 patients (13%) were on tablet morphine 60 mg and 80 mg/day. One patient (7%) was on injection tramadol 300 mg/day. There was statistically significant decrease in mean analgesic consumption at all time intervals (p< 0.05)(Table 3). One patient remained completely pain free till 21 days and needed supplemental analgesics thereafter but VAS scores remained high in spite of opioids. So he was planned for a repeat block. He was pain free until death after repeat block. Repeat block was given in another patient whose VAS score rose to 5 at 2nd month inspite of supplemental analgesics. Supplemental analgesics were required at 24th hour in two patients, at one month in one patient and at second month in another patient. Eleven patients (73%) had complete pain relief for an average duration of 71.6 days and four patients (27%) had only partial pain relief for an average duration of 35.5 days (Table 4).
Table 3:Average analgesic consumption after CPN. Data given as Mean ± SD (Range)
|
Medicine |
Before CPN |
After CPN 24hr |
After CPN 1wk |
After CPN 1mth |
After CPN 2mth |
After CPN 3mth |
| Diclofenac (mg/day) |
106.25 ± 17.68* |
0.00* |
0* |
0* |
0* |
0* |
|
(100-150) |
||||||
| p-value |
0.00000 |
0.00000 |
0.00019 |
0.00145 |
0.00990 |
|
| PCM (mg/day) |
1125 ± 0$ |
80.3 ± 300.67$ |
160.7 ± 408.5$ |
204.5 ± 455$ |
250 ± 496$ |
281.25 ± 520.7$ |
|
(0-1125) |
(0-1125) |
(0-1125) |
(0-1125) |
(0-1125) |
(0-1125) |
|
| p-value |
0.00000 |
0.00000 |
0.00003 |
0.00037 |
0.00127 |
|
| Morphine (mg/day) |
70.0 ± 14.14# |
0# |
0# |
0# |
0# |
0# |
|
(60-80) |
||||||
| p-value |
0.04517 |
0.04517 |
0.04517 |
0.04517 |
0.04517 |
|
| Tramadol (mg/day) |
125 ± 48.41¥ |
7.5 ± 29.05¥ |
15 ± 39.58¥ |
18.75 ± 43.79¥ |
22.5 ± 47.43¥ |
25.00 ± 49.61¥ |
|
(112.5-300) |
(0-112.5) |
(0-112.5) |
(0-112.5) |
(0-112.5) |
(0-112.5) |
|
| p-value |
0.00000 |
0.00001 |
0.00017 |
0.00115 |
0.00284 |
Table 4: Duration of pain relief
| Duration (days) |
Mean ± SD |
Range |
| Complete pain relief |
71.63 ± 23.28 |
20-90 days |
| Partial pain relief |
35.5 ± 31.10 |
7-90 days |
| Patient follow-up |
67.33 ± 32.73 |
7-90 days |
DISCUSSION
Posterior approach is associated with neurological complicationsresulting from posterior spread of neurolytic towards the lumbar plexus in 1% of patients.12The anterior approach involves placing the drug anterior to the crura of diaphragm and aorta leading to reduced procedure time, and use of a smaller volume of drug. It also avoids puncture of the aorta, ensures placement of the tip of the needle anterior to the spinal arteries and spinal canal
The variations include the use offluoroscopy percutaneous approach,ultrasound (USG)13,14 endoscopic ultrasound (EUS)15,16computed tomography4,7,17 and magnetic resonance image (MRI)18 guided CPN.
In our study neurolysis was performed using 35-40 ml of 50% alcohol. Eleven patients (73%) had effective pain relief (VAS score 3 or less) upto three months or until death after neurolysis. Four patients (27%) did not have satisfactory pain relief after CPN but the pain scores were still lower than baseline. The success rate was lower in the studies by Lieberman et al (55%)31, Romanelli et al (57%)4, Gimenez et al (61%)10, Gress et al (55%)11, Gunaratnam et al (54%).12 Eleven patients (73%) of patients had complete pain relief lasting for an average duration of 71.63 days and four patients (27%) had partial pain relief for average duration of 35.5 days which is comparable to studies by Matamala et al,13Wiersema et al14 and Tran et al.8 Three of them had undergone surgical intervention. One had liver metastasis secondary to carcinoma rectum. Average daily analgesic consumption was statistically significant at all follow-up intervals up to 3 months, signifying a good pain relief. The efficacy diminished at 8-12 weeks after which pain scores in patients not receiving adjuvant therapy trended upward. Three patients were pain free till death though they could not complete their follow-up of 3 months.The extension of cancer invasion and eventual postoperative changes may compromise the outcomes, by limiting the spread of the neurolytic agent around the celiac trunk thereby explaining the partial relief obtained.10
Gimenez et al performed CPN in 38 patients (34 of abdominal tumors and 4 of chronic pancreatitis) under ultrasonographic guidance. There was complete pain relief in 61% patients at one week and at six months. After one year pain was totally relieved in 39%, partially relieved in 52% and unchanged in 9% patients, signifying a good outcome after using ultrasound to localize coeliac plexus. All the patients in whom the pain was partially relieved or unchanged had undergone surgical procedure in the area where CPN was performed. They have attributed it to the hindrance of spread of neurolytic solution around the coeliac plexus due to postoperative changes.10 They have not evaluated the daily analgesic usage after CPN.
Romanelli et al performed CPN under CT guidance in 17 patients of chronic abdominal pain of coeliac ganglion origin. Eleven patients (79%) had some relief of pain, out of whom 57% had complete relief. The complete data on use of pain medications was available in ten patients in whom the mean daily analgesic usage decreased from 17-100% (Mean 58%) relative to preprocedure dosage. Significant benefit was not observed in three patients with the diagnosis of chronic pancreatitis in two of them.41 They have not mentioned the follow-up intervals. The timings of decrease in the mean VAS as well as analgesic usage are not mentioned in their study.
Gunaratnam et al prospectively studied 58 patients who underwent EUS CPN for pain secondary to inoperable pancreatic cancer. Neurolysis was performed by injecting ten ml of 0.25% bupivacaine and ten ml (98%) alcohol on both sides of the celiac region. Forty-five patients (78%) experienced a drop in pain scores two weeks after CPN. This effect was sustained for 24 weeks. However only 31 patients (54%) experienced a decline of more than two points, a measure of improvement that some consider necessary to signify efficacy. However, patients who received chemotherapy alone or in combination with radiotherapy experienced more pain relief. Though opioid administration increased throughout the study, the increase was not statistically significant. The efficacy of EUS guided CPN diminished at 8-12 weeks. Complications reported were mild and transient- hypotension (20%), diarrhea (17%) and pain exacerbation (9%).12
Matamala et al used ultrasound to confirm needle placement in nine patients of chronic pancreatic pain and found total pain relief in seven patients after two weeks and in five patients after six months. Two patients did not experience any pain relief.13
Gress et al performed EUS guided CPB in 90 patients of chronic pancreatitis. They injected 10 ml bupivacaine 0.5% and 3 ml (40 mg) of triamcinolone on each side of the coeliac plexus. A significant improvement in overall pain scores occurred in 55% of patients. The mean pain score decreased from 8 to 2 post EUS coeliac plexus block at both four and eight weeks follow-up (p < 0.05). In 26% of patients there was persistent benefit beyond 12 weeks, and 10% still had persistent benefit at 24 weeks including three patients who had long term pain control between 35 and 48 weeks. The lower success rate in their study could be attributed to the use of steroids and all the patients being of chronic pancreatitis only. They also concluded that EUS coeliac block was more cost efficient.11
Das and Chapman performed sonography guided CPB using local anestheticin nine patients before hepatobiliary interventions. The position of needle tip was also confirmed by fluoroscopy and considered satisfactory if found overlying body of L1. Good pain relief was reported in 85% of patients. The time taken to perform block was reported to be less than five min, whereas it was 7.73 min in our study.19
Success rate reported with classic technique rangesfrom 44-94% lasting from one month to one year20 and varies 54-94% using anterior approach.12, The failures could be attributed to anatomical variations in position of the coeliac plexus, inadequate spread of neurolytic agent, fibrosis due to previous surgery, presence of metastasis, extension of primary tumor involving the abdominal wall not innervated by coeliac plexus. Though few studies have attributed it to poor placement of needle and alternate pain pathways. CPN relieves only the visceral component of pain, decreases pain and analgesic requirement but it is not sufficient in terms of complete long term pain relief,21 so it is considered by some authors to be the optimal treatment.We have not observed anycomplication related to the procedure. The spread using the anterior approach covers the coeliac plexus without involving the retrocrural region and psoas compartment containing the sympathetic chain and lumbar plexus. This reduces the risk of neurological complications. The most collateral effects are related to sympathetic block. Incidence of diarrhea was lower (6.67%) in our study as compared to Matamala et al (66%) and Gimenez et al (13%). Transient hypotension occurred in one patient (6.67%). Needle broke in one patient at the junction of hub and needle shaft.Almost whole needle had been introduced in an attempt to reposition the needle close to origin of coeliac artery and as depth of coeliac trunk on USG was 13.8 cm. Only stellate of the needle came out along with hub of the needle. It could be attributed to the manufacturing defect of chiba needle. This patient was explored surgically and needle removed. Though this approach involves penetration of abdominal viscera and blood vessels, reported incidence of complications is low as sonography allows blood vessels to be visualized.
The CT and MRI provides a better quality image than ultrasound, but are costly and time consuming, whereas ultrasound guidance is quicker and more economical.However, sonographic approach requiresindividual skills and training in interventional radiology.Therelevant drawback of US-guidance is the poor visualization of thin needles during their progression, with the potential of the needle’s improper positioning.22
LIMITATIONS
Limitations included uncontrolled nature which allowed a limited enrollment, provided inadequate power to permit firm conclusions. We did not evaluate any cost comparisons. Depth of coeliac trunk was not assessed in all patients.Patients were followed up only for three months .
CONCLUSION
To conclude sonographic CPN is an efficient, safe, and fast method for relieving pain in the upper abdominal malignancy. The use of ultrasound helps inreal time needle placement, and helps to examine the drug spread around aorta.Hence, sonographic anterior approach can be used in patients of upper abdominal malignancy for coeliac plexus neurolytic block. However,multi-center controlled investigations with larger sample size to evaluate cost-effectiveness, clinical outcomes, and effect on quality of life are necessary before the use of sonographic guided CPN can be considered a cost effective alternative.
REFERENCES
- Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. CT-guided celiac plexus neurolysis: A review of anatomy, indications, technique, and tips for successful treatment. Radiographics 2011;31:1599-1621. [PubMed][Free full text]doi: 10.1148/rg.316115526.
- Kappis M. Sensibilitat und lokaleAnaesthesieimChirurgischenGebret der BauchhohlemitbesondererBerucksichtigung der Splanchnicusacrasthesie. BeitrKlinChir 1919;115:161-75
- Mercadante S, Nicosia F. Celiac plexus block: a reappraisal. RegAnesth and Pain Med 1998;23(1):37-48 [PubMed]
- Romanelli DF, Beckmann CF, Heiss FW. Celiac plexus block: efficacy and safety of the anterior approach. Am J Roentgenol. 1993;160(3):497-500 [PubMed][Free full text] doi: 2214/ajr.160.3.8430543
- Herpels V, Kurdziel JC, Dondelinger RF. Percutaneous CT guided nerve block of the celiac plexus and splanchnic nerves. Ann Radiol (Paris) 1988;31(5):291-6. [PubMed]
- Haaga JR, Kori SH, Eastwood DW, Borkowski GP. Improved technique for CT guided celiac ganglia block. Am J Radiol 1984;142(6):1201-4. [PubMed][Free full text]
- Montero Matamala A, Vidal Lopez F, Inaraja Martinez L. Percutaneous approach to the celiac plexus using CT guidance. Pain 1988:34(3):285-8. [PubMed]
- Tran QH, Urayama S, Meyers FJ. Endoscopic ultrasound guided celiac plexus neurolysis for pancreatic cancer pain: a single institution experience and review of literature. J Support Oncol. 2006 Oct;4(9):460-2. [PubMed]
- De Cicco M, Matovic M, Bortolussi R, Coran F, Fantin D, Fabiani F, et al. Celiac plexus block: injectate spread and pain relief in patients with regional anatomic distortions. Anesthesiology 2001;94(4):561-5. [PubMed][Free full text]
- Giménez A, Martínez-Noguera A, Donoso L, Catalá E, Serra R. Percutaneousneurolysis of celiac plexus via the anterior approach with sonographic guidance. Am J Roentgenol. 1993;161(5):1061-3. [PubMed][Free full text]
- Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single centre experience. Am J Gastroenterol. 2001;96(2):409-16. [PubMed]
- Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. GastrointestEndosc. 2001;54(3):316-24. [PubMed]
- Montero Matamala A, Vidal Lopez F, Aguilar Sanchez L, Donoso Bach L. Percutaneous anterior approach to coeliac plexus using ultrasound. Br J Anaesth. 1989;62(6):637-40 [PubMed][Free full text]doi:1093/bja/62.6.637
- Garcia RG, Maurano A, Santos MMD, Filho CLD, Macedo ALV, Neto MJF, Funari Ultrasound guided percutaneous celiac plexus neurolysis using the anterior transgastric approach and continuous flow apneic ventilation: case report. Einstein 2009;7:361-4 [Free full text]
- Wiersema MJ, Kochman LM, Cramer HM, Tao LC,Wiersema LM. Endosonography guided real time fine needle aspiration biopsy. GastrointestEndosc. 1994;40(6):700-7 [PubMed]
- Soweid AM, Azar C. Endoscopic ultrasound guided celiac plexus neurolysis. World J GastrointestEndosc 2010;2(6):228-31[PubMed][Free full text]doi: 10.4253/wjge.v2.i6.228.
- Singler RC. An improved technique for alcohol neurolysis of celiac plexus. Anesthesiology 1982;56(2):137-41[PubMed][Free full text]
- Hol PK, Kvarstein G, Viken O, Smedby O, Tonnessen TI. MRI-guided celiac plexus block. J MagnReson Imaging. 2000;12(4):562-4 [PubMed]
- Das KM, Chapman AH.Sonographically guided coeliac plexus block. ClinRadiol. 1992;45:401–3
- Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: ameta analysis. AnesthAnalg. 1995;80:290-5.[PubMed]
- Ischia S, Ischia A, Polati E, Finco G. Three posterior percutaneous celiac plexus block techniques: A prospective randomized study in 61 patients with pancreatic cancer pain. Anesthesiology 1992;76(4):534-40.[PubMed][Free full text]
- Waldman SD, Patt RB. Splanchnic and celiac plexus nerve block. In: Waldman SD, editor. Pain Management. 1st Philadelphia: Saunders Elsevier;2007 p 1265-8