Abdulkader A. Al-Shakour1, Hussein A. Khalid2, Naser Ali Naser3
Author affiliations:
Background & objective: Netrin-1 is commonly recognized as a neural guidance cue that has been suggested to play a role in pancreas development. Multiple studies have reported on the regenerative, angiogenic, and anti-inflammatory properties of netrin-1 in various tissues. In hyperglycemia, netrin-1 may support insulin secretion and reduce inflammation. This study was aimed to investigate the correlation of serum Netrin-1 with Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
Methodology: This study comprised a total of 81 patients diagnosed with type 2 diabetes mellitus (T2DM) and 79 apparently healthy individual as controls. For each participant' following an overnight fasting, samples of blood were taken. Biochemical parameters were estimated including glycated hemoglobin, fasting blood glucose, serum insulin, and serum netrin-1 levels.
Result: This study revealed that T2DM patients had significantly higher serum netrin-1 levels than the control group. There was a significant positive correlation between netrin-1 and HOMA-IR.
Conclusion: The mean serum concentration of netrin-1 was significantly higher in type 2 diabetes mellitus patients than in healthy individuals. There is a positive correlation between insulin resistance and netrin-1 in type 2 diabetes mellitus. Further studies involving larger sample sizes are needed to clarify the real relationship and to improve reliability and replicability and to provide an insight to the pathogenesis, diagnosis, prevention, and treatment of type 2 diabetes mellitus.
Abbreviations: ADA - American Diabetes Association DCC - Deleted Receptors in Colorectal Cancer; HOMA-IR - Homeostatic Model Assessment of Insulin Resistance; T2DM - Type 2 Diabetes Mellitus; UNC5 - Uncoordinated 5;
Keywords: Diabetes Mellitus; Hyperglycemia; Insulin Resistance; Netrin-1
Citation: Al-Shakour AA, Khalid HA, Naser NA. Serum netrin-1 level and insulin resistance in type 2 diabetes mellitus. Anaesth. pain intensive care 2024;28(2):341−346; DOI: 10.35975/apic.v28i2.2422
Received: January 07, 2024; Revised: February 01, 2024; Accepted: February 08, 2024
Type 2 diabetes mellitus (T2DM) is the most prevalent form of diabetes, which is a metabolic disease of multiple etiology, and a steadily growing number of people acquire the disease around the world.1 Due to the aging of nations, more than 590 million people will be diagnosed with this disease by 2035, making it a global epidemic.2 The main factors contributing to the development of T2DM are the deterioration of β-cells and the presence of insulin resistance.3 After β-cell dysfunction and loss, there is insufficient insulin production to compensate for decreased peripheral insulin sensitivity, which leads to persistent hyperglycemia.3 The pancreatic β cells manufacture insulin, a peptide hormone consisting of 51 amino acids. Insulin is a hormone with pluripotent properties, meaning it can elicit various biological reactions and play a crucial role in various biological processes.4 It enhances the transportation of glucose between muscle and adipose tissue, regulates the functioning of enzymes within cells, and controls the transcription of specific genes.4 Insulin resistance is the inability of insulin to support metabolic processes and is a medical condition where there is a change in the dose response curve, resulting in a reduction in the body's response to insulin.5 A deficient response to insulin can be seen either across the entire range of insulin concentrations or specifically at minimal levels of the hormone.5
Netrin-1 is originally considered an axon guidance protein, with a structure similar to laminine.6 The complete transcript has seven exons and is transformed into a protein with a length of 604 amino acids with molecular mass of 50–75 kD.3,4 The ‘Deleted Receptors in Colorectal Cancer’ (DCC) and the ‘Uncoordinated 5’ (UNC5) receptors are two widely established receptor families that regulate the biological functions of netrin.3,7 Netrin-1 plays a vital role in the initial development of different tissues, including the nervous system, vascular system, pulmonary system, pancreatic system, muscular system, and mammary gland.4 Netrin-1 has also been linked to tissue regeneration, control of inflammatory conditions, and leukocyte migration in peripheral organs.3 Both endocrine and exocrine cells expressed netrin-1.
In the pancreas, neogenin was highly expressed, which suggests that netrin-1 is involved in pancreatic development, remodeling of tissue, migration of islet cells, and also regeneration.8 When the β-cells were treated with external netrin-1, caspase-3 was discovered to be downregulated.9 Neogenin and UNC5-A receptors are also decreased along with the decrease in caspase-3 cleavage. However, neogenin and UNC5 receptors have shown the capacity to trigger cell death without netrin while inhibiting it when they bind with netrin-1; this discovery highlights netrin's contribution to β-cell prosurvival.10 One of the main causes of diabetes is inflammation, and studies have shown that netrin-1 reduces inflammation by interacting with chemokines and inflammatory cytokines, as a result, serum netrin-1 levels may have an association with insulin resistance.11,12
This study was aimed to investigate the relationship between serum netrin-1 and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
It was a case-control research study, carried out in Al-Muane Teaching Hospital, in the southern Iraqi province (Basrah), from December 2022 to June 2023. A total of 160 participants were divided into 81 T2DM patients and 79 healthy controls, matched for age and sex with the patients. All of the participants attended the hospital, either for routine checkups or medical consultations.
2.2. Study population
2.2.1. Cases: According to the American Diabetes Association (ADA) guidelines for diabetes diagnosis, a total of 81 participants have been identified as having T2DM for at least one year, and the diagnosis was the following: “a FPG level of ≥126 mg/dL (7.0 mmol/L)”, Or “a 2-h PG level ≥200 mg/dL (11.1mmol/L), Or HbA1c level ≥6.5% (48 mmol/mol).13
2.2.2. Control: The control group was included 79 individuals who were age and sex matched with patients. These people seemed to be healthy a careful clinical examination showed that they were in good health, and they agreed to participate in this study.
2.2.3. Exclusion Criteria: Patient with type 1 diabetes mellitus, Pregnant and lactating women, Patients with chronic liver diseases, patients with renal impairment, patients with hormone replacement therapy, Patient with congestive heart failure.
2.3. Sample collection
Five ml of venous blood was drawn after an overnight fast of at least eight hours from each participant. Tow mL was dispensed into a EDTA tube for the estimation the HbA1c% by ion exchange high-performance liquid chromatography using Variant II Turbo HbAlc Kit-2.0, supplied by Bio-Rad, USA, (REF 220-0220). The remainder of the blood was collected in a serum separator tube (SST). Half of the serum was used for the estimation of fasting blood sugar (FBS) and serum insulin (kits from Roche Diagnostics Gmbh, Germany, REF 04404483190and REF 12017547122 respectively) by using a fully automated chemistry analyzer, Cobas C311. The remaining portion was placed into Eppendorf tubes and preserved at -20°C for later determination of serum netrin-1. Estimation of netrin-1 was done by a sandwich enzyme-linked immunosorbent assay (ELISA) kit (Sunlong Biotech, China, REF SL1249Hu). The inter-assay precision value was less than 10%, but the intra-assay precision value was less than 8%.
Insulin resistance Models (Matthews et al 1985).14
a) Normal value ≤ 2.5 where IR is insulin resistance.
Statistical analysis
The data from this study were analyzed with the Statistical Package for Social Science (SPSS) version 28 program. The results are given as mean ± standard deviation (SD) and numbers and percentage. For continuous data, the Independent t-test is utilized to compare two different groups. Chi-square (x2 test) is used to compare proportions of two or more groups in categorical data. P-value of 0.05 is considered as the highest limit for significance.
The sociodemographic characteristics and biochemical parameters of the patients and controls have been given in Table 1. The data revealed that the average FBS in cases (229.44 ± 85.20 mg) was significantly higher than that of controls (103.51 ± 13.38 mg) (P < 0.001). In addition, the mean HbA1c% was significantly higher among cases than among the controls (8.59 ± 2.00 and 5.05 ± 0.55 respectively) (P < 0.001).
The mean insulin level was significantly higher among cases than the controls (16.58 ± 12.45 and 7.63 ± 4.46 respectively) (P < 0.001). Regarding HOMA-IR, diabetic patients had a significant increase in insulin resistance in comparison to controls (9.23 ± 6.25 and 1.96 ± 1.23 respectively), with a highly significant difference (P < 0.001). The mean value of serum netrin-1 was significantly higher in patients in comparison to the control group (185.90 ± 34.67 and 96.57 ± 12.12 respectively) (P < 0.001).
Comparison of the serum netrn-1 level in relation to disease duration, FBS, HbA1c, and serum insulin were shown in table 2. The results showed that the mean value of serum netrin-1 was insignificantly higher in people who had the duration of disease for more than 5 y. With respect to FBS, the results showed that patients with FBS levels exceeding >130 mg/dL have higher netrin-1 levels as compared to those with FBS levels of ≤130 mg/dL (P < 0.001). Also this study revealed that patients with poor glycemic control (HbA1c > 8%) had higher levels of serum netrin-1 as compared to those with good glycemic control (HbA1c7-8%), and optimal glycemic control (HbA1c <7%) respectively. With respect to serum insulin level patients with insulin levels ≥ 20 mIU/mL had higher mean value of netrin-1 as compared to those with serum insulin levels < 20 mIU/mL (P < 0.001).
Netrin-1 is a member of the laminin-related proteins of axon guidance and has been shown to have a variety of roles via its two traditional receptor families, including deleted receptors in colorectal cancer (DCC) and uncoordinated 5 (UNC5).15 Netrin-1, which functions as a neuro-immune signaling molecule, has a significant impact on embryonic pancreas development. Regeneration, tissue remodeling, islet cell migration, and pancreatic morphogenesis are all impacted by netrin-1.16,17
This study found that individuals with T2DM had significantly higher mean serum netrin-1 values compared to controls, which is likely due to a compensatory reaction to the disease. Several other studies revealed similar finding.18–22
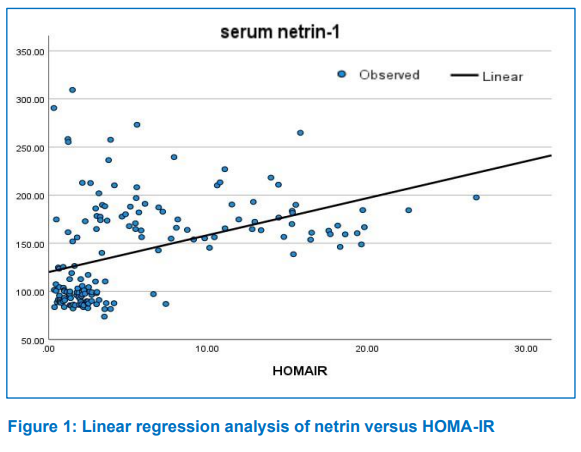
The current study revealed that there was significant positive correlation between patients age and serum netrin-1 level (r = 0.054, P = 0.497). Also, the patients mean value of serum netrin-1 levels were greater than those of the control group at the same age group, with a highly significant difference (P < 0.001). These results were consistent with those of other studies.23,24,25
Also, there was a significant positive relationship between serum netrin-1 levels and HbA1c. Similar finding also stated by other studies.18–22 In contrary some others studies reported controversial result.26, 27,28
This variation in relation to the association between T2DM and serum netrin-1 is indistinct, which could be imputed to diversity with respect to specimen collection, sample size, ethnic group, other sociodemographic differences of the investigated population, and estimation method. Additionally, the result of this study showed a significant positive association between serum glucose levels and HbA1c with serum netrin-1 levels (r = 0.568, P < 0.001; r = 0.628, P = 0.001 respectively). These findings were in concordance with the results of other studies.8,21,29,30,31 However, some other studies demonstrated a controversial result.25,26,28 Gao et al.3 stated that netrin-1 stimulates insulin release from the pancreas by accelerating calcium inflow to the β-cell and the generation of the cAMP. The observation of this study may provide an insight that serum netrin-1 level may be used as an indicator for the control of T2DM.
The HOMA-IR is a measurement that assesses insulin resistance levels in the body and high value of HOMA-IR refers to the decreased ability of cells to respond properly to insulin and glucose load. The development of diabetes and other metabolic disorders can be delayed with an early diagnosis of insulin resistance. The success of diabetes treatment can also be tracked by clinicians using the HOMA, who can then modify the treatment strategy as necessary.6 The study revealed a positive correlation between serum netrin-1and HOMA-IR. This result was in agreement with finding of Natura G, who stated that netrin-1 could coordinate inflammation, which might lead to negative regulation of insulin secretion from β-cell and participate into β-cell failure.32 Several studies revealed similar findings.8,30,33,34,35 Ramkhelawon et al.34 stated that insulin resistance perturbs the netrin-1 circulation and netrin-1 over expression supports imperfection of adipose tissue immigration and macrophage retention, which might possibly enhance the promotion of chronic inflammation, metabolic dysfunction and insulin resistance. However, other studies revealed controversial results.26 They stated that netrin-1 has a retention cue for macrophage in visceral adipose tissue which possibly enhance the progression of the chronic inflammation and the subsequent development of insulin resistance, that happens in T2DM.26 It has been concluded that netrin-1 may have a regulatory effect on inflammatory process which might lead to negative regulation of insulin secretion and may have a role in dysfunction of β-cell.32
In conclusion the contradictory results with respect to the association between netrin-1 and type 2 diabetes mellitus, and the association between netrin-1 and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) may require further studies involving a larger sample size to clarify the real relationship and to improve reliability and replicability and to provide an insight to the pathogenesis, diagnosis, prevention, and treatment of type 2 diabetes mellitus.
6. Data availability
The numerical data generated during this research is available with the authors.
7. Acknowledgement
We gratefully thank Department of Biochemistry, College of Medicine, University of Basrah, for their generous help in completion of this research.
8. Conflict of interest
The study utilized the hospital resources only, and no external or industry funding was involved.
9. Authors’ contribution
AAA: Drafting the manuscript
HAK, NAN: Conduction of the study work and manuscript editing
MMMA: Evaluation and sending the manuscript
Author affiliations:
- Abdulkader A. Al-Shakour, Department of Biochemistry, College of Medicine, University of Basrah, Basrah, Iraq; E-mail: Abdulkader1010@yahoo.com
- Hussein A. Khalid, Department of Biochemistry, College of Medicine, University of Basrah, Basrah, Iraq; E-mail: Hussein.wkhalid0@gmail.com
- Naser Ali Naser, Al-Faiha’a Teaching Hospital, Basra, Iraq; E-mail: nasor.ali0@gmail.com
ABSTRACT
Background & objective: Netrin-1 is commonly recognized as a neural guidance cue that has been suggested to play a role in pancreas development. Multiple studies have reported on the regenerative, angiogenic, and anti-inflammatory properties of netrin-1 in various tissues. In hyperglycemia, netrin-1 may support insulin secretion and reduce inflammation. This study was aimed to investigate the correlation of serum Netrin-1 with Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
Methodology: This study comprised a total of 81 patients diagnosed with type 2 diabetes mellitus (T2DM) and 79 apparently healthy individual as controls. For each participant' following an overnight fasting, samples of blood were taken. Biochemical parameters were estimated including glycated hemoglobin, fasting blood glucose, serum insulin, and serum netrin-1 levels.
Result: This study revealed that T2DM patients had significantly higher serum netrin-1 levels than the control group. There was a significant positive correlation between netrin-1 and HOMA-IR.
Conclusion: The mean serum concentration of netrin-1 was significantly higher in type 2 diabetes mellitus patients than in healthy individuals. There is a positive correlation between insulin resistance and netrin-1 in type 2 diabetes mellitus. Further studies involving larger sample sizes are needed to clarify the real relationship and to improve reliability and replicability and to provide an insight to the pathogenesis, diagnosis, prevention, and treatment of type 2 diabetes mellitus.
Abbreviations: ADA - American Diabetes Association DCC - Deleted Receptors in Colorectal Cancer; HOMA-IR - Homeostatic Model Assessment of Insulin Resistance; T2DM - Type 2 Diabetes Mellitus; UNC5 - Uncoordinated 5;
Keywords: Diabetes Mellitus; Hyperglycemia; Insulin Resistance; Netrin-1
Citation: Al-Shakour AA, Khalid HA, Naser NA. Serum netrin-1 level and insulin resistance in type 2 diabetes mellitus. Anaesth. pain intensive care 2024;28(2):341−346; DOI: 10.35975/apic.v28i2.2422
Received: January 07, 2024; Revised: February 01, 2024; Accepted: February 08, 2024
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is the most prevalent form of diabetes, which is a metabolic disease of multiple etiology, and a steadily growing number of people acquire the disease around the world.1 Due to the aging of nations, more than 590 million people will be diagnosed with this disease by 2035, making it a global epidemic.2 The main factors contributing to the development of T2DM are the deterioration of β-cells and the presence of insulin resistance.3 After β-cell dysfunction and loss, there is insufficient insulin production to compensate for decreased peripheral insulin sensitivity, which leads to persistent hyperglycemia.3 The pancreatic β cells manufacture insulin, a peptide hormone consisting of 51 amino acids. Insulin is a hormone with pluripotent properties, meaning it can elicit various biological reactions and play a crucial role in various biological processes.4 It enhances the transportation of glucose between muscle and adipose tissue, regulates the functioning of enzymes within cells, and controls the transcription of specific genes.4 Insulin resistance is the inability of insulin to support metabolic processes and is a medical condition where there is a change in the dose response curve, resulting in a reduction in the body's response to insulin.5 A deficient response to insulin can be seen either across the entire range of insulin concentrations or specifically at minimal levels of the hormone.5
Netrin-1 is originally considered an axon guidance protein, with a structure similar to laminine.6 The complete transcript has seven exons and is transformed into a protein with a length of 604 amino acids with molecular mass of 50–75 kD.3,4 The ‘Deleted Receptors in Colorectal Cancer’ (DCC) and the ‘Uncoordinated 5’ (UNC5) receptors are two widely established receptor families that regulate the biological functions of netrin.3,7 Netrin-1 plays a vital role in the initial development of different tissues, including the nervous system, vascular system, pulmonary system, pancreatic system, muscular system, and mammary gland.4 Netrin-1 has also been linked to tissue regeneration, control of inflammatory conditions, and leukocyte migration in peripheral organs.3 Both endocrine and exocrine cells expressed netrin-1.
In the pancreas, neogenin was highly expressed, which suggests that netrin-1 is involved in pancreatic development, remodeling of tissue, migration of islet cells, and also regeneration.8 When the β-cells were treated with external netrin-1, caspase-3 was discovered to be downregulated.9 Neogenin and UNC5-A receptors are also decreased along with the decrease in caspase-3 cleavage. However, neogenin and UNC5 receptors have shown the capacity to trigger cell death without netrin while inhibiting it when they bind with netrin-1; this discovery highlights netrin's contribution to β-cell prosurvival.10 One of the main causes of diabetes is inflammation, and studies have shown that netrin-1 reduces inflammation by interacting with chemokines and inflammatory cytokines, as a result, serum netrin-1 levels may have an association with insulin resistance.11,12
This study was aimed to investigate the relationship between serum netrin-1 and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
2. METHODOLOGY
It was a case-control research study, carried out in Al-Muane Teaching Hospital, in the southern Iraqi province (Basrah), from December 2022 to June 2023. A total of 160 participants were divided into 81 T2DM patients and 79 healthy controls, matched for age and sex with the patients. All of the participants attended the hospital, either for routine checkups or medical consultations.
2.2. Study population
2.2.1. Cases: According to the American Diabetes Association (ADA) guidelines for diabetes diagnosis, a total of 81 participants have been identified as having T2DM for at least one year, and the diagnosis was the following: “a FPG level of ≥126 mg/dL (7.0 mmol/L)”, Or “a 2-h PG level ≥200 mg/dL (11.1mmol/L), Or HbA1c level ≥6.5% (48 mmol/mol).13
2.2.2. Control: The control group was included 79 individuals who were age and sex matched with patients. These people seemed to be healthy a careful clinical examination showed that they were in good health, and they agreed to participate in this study.
2.2.3. Exclusion Criteria: Patient with type 1 diabetes mellitus, Pregnant and lactating women, Patients with chronic liver diseases, patients with renal impairment, patients with hormone replacement therapy, Patient with congestive heart failure.
2.3. Sample collection
Five ml of venous blood was drawn after an overnight fast of at least eight hours from each participant. Tow mL was dispensed into a EDTA tube for the estimation the HbA1c% by ion exchange high-performance liquid chromatography using Variant II Turbo HbAlc Kit-2.0, supplied by Bio-Rad, USA, (REF 220-0220). The remainder of the blood was collected in a serum separator tube (SST). Half of the serum was used for the estimation of fasting blood sugar (FBS) and serum insulin (kits from Roche Diagnostics Gmbh, Germany, REF 04404483190and REF 12017547122 respectively) by using a fully automated chemistry analyzer, Cobas C311. The remaining portion was placed into Eppendorf tubes and preserved at -20°C for later determination of serum netrin-1. Estimation of netrin-1 was done by a sandwich enzyme-linked immunosorbent assay (ELISA) kit (Sunlong Biotech, China, REF SL1249Hu). The inter-assay precision value was less than 10%, but the intra-assay precision value was less than 8%.
| HOMA –IR a | (fasting glucose x fasting insulin) ÷ 405 ; insulin expressed in μIU/ml, glucose in mg/dl. |
Insulin resistance Models (Matthews et al 1985).14
a) Normal value ≤ 2.5 where IR is insulin resistance.
Statistical analysis
The data from this study were analyzed with the Statistical Package for Social Science (SPSS) version 28 program. The results are given as mean ± standard deviation (SD) and numbers and percentage. For continuous data, the Independent t-test is utilized to compare two different groups. Chi-square (x2 test) is used to compare proportions of two or more groups in categorical data. P-value of 0.05 is considered as the highest limit for significance.
3. RESULTS
The sociodemographic characteristics and biochemical parameters of the patients and controls have been given in Table 1. The data revealed that the average FBS in cases (229.44 ± 85.20 mg) was significantly higher than that of controls (103.51 ± 13.38 mg) (P < 0.001). In addition, the mean HbA1c% was significantly higher among cases than among the controls (8.59 ± 2.00 and 5.05 ± 0.55 respectively) (P < 0.001).
The mean insulin level was significantly higher among cases than the controls (16.58 ± 12.45 and 7.63 ± 4.46 respectively) (P < 0.001). Regarding HOMA-IR, diabetic patients had a significant increase in insulin resistance in comparison to controls (9.23 ± 6.25 and 1.96 ± 1.23 respectively), with a highly significant difference (P < 0.001). The mean value of serum netrin-1 was significantly higher in patients in comparison to the control group (185.90 ± 34.67 and 96.57 ± 12.12 respectively) (P < 0.001).
| Table 1: Sociodemographic and biochemical parameters of patients and control. | ||||
| Variables | Cases
(n = 81) |
Controls
(n = 79) |
P value | |
| Age (y) | 53.60 ± 9.10 | 52.60 ± 9.67 | NS | |
| Age (y) | 30-39 y | 7 (8.6) | 8 (10.1) | NS |
| 40-49 y | 18 (22.2) | 19 (24.1) | ||
| 50-59 y | 29 (35.8) | 29 (36.7) | ||
| 60-69 y | 25 (30.9) | 20 (25.3) | ||
| >70 y | 2 (2.5) | 3(3.8) | ||
| Gender | Male | 38 (46.9) | 39 (49.4) | NS |
| Female | 43 (53.1) | 40 (50.6) | ||
| FBS (mg/dL) | 229.44 ± 85.20 | 103.51 ± 13.38 | < 0.001 | |
| HbA1c (%) | 8.59 ± 2.00 | 5.05 ± 0.55 | < 0.001 | |
| Insulin Level (mIU/mL) | 16.58 ± 12.45 | 7.63 ± 4.46 | < 0.001 | |
| HOMA-IR | 9.23 ± 6.25 | 1.96 ± 1.23 | < 0.001 | |
| Netrin-1 (pg/ml) | 185.90 ± 34.67 | 96.57 ± 12.12 | < 0.001 | |
| Data presented as mean ± SD or n (%); P < 0.05 is considered significant | ||||
Comparison of the serum netrn-1 level in relation to disease duration, FBS, HbA1c, and serum insulin were shown in table 2. The results showed that the mean value of serum netrin-1 was insignificantly higher in people who had the duration of disease for more than 5 y. With respect to FBS, the results showed that patients with FBS levels exceeding >130 mg/dL have higher netrin-1 levels as compared to those with FBS levels of ≤130 mg/dL (P < 0.001). Also this study revealed that patients with poor glycemic control (HbA1c > 8%) had higher levels of serum netrin-1 as compared to those with good glycemic control (HbA1c7-8%), and optimal glycemic control (HbA1c <7%) respectively. With respect to serum insulin level patients with insulin levels ≥ 20 mIU/mL had higher mean value of netrin-1 as compared to those with serum insulin levels < 20 mIU/mL (P < 0.001).
| Table 2: Distributions of serum netrin-1 according to the duration of disease, FBS, HbA1c, and serum insulin. | |||
| Parameter | Netrin-1 (pg ̷ml) | P-value | |
| Duration of disease | ≤ 5 y | 183.54 ± 25.60 | NS |
| > 5 y | 186.68 ± 37.32 | ||
| FBS (mg/dL) |
≤130 | 108.23 ± 37.38 | < 0.001 |
| ˃130 | 183.87 ± 33.34 | ||
| HbA1c | Good control < 7% | 113.20 ± 44.24 | < 0.001 |
| Fair control 7-8% | 180.64 ± 30.32 | ||
| Poor control ˃ 8% | 181.80 ± 51.81 | ||
| Insulin Level (mIU/mL) | < 20 | 135.10 ± 52.50 | < 0.001 |
| ≥20 | 172.05 ± 35.84 | ||
| Data presented as mean ± SD or n (%); P < 0.05 is considered significant | |||
| Table 3: Pearson correlation of serum netrin-1 levels with the biochemical parameters in the study population. | ||
| Variables | Netrin-1
(pg ̷ml) |
|
| FBS
(mg ̷ dL) |
Correlation Coefficient | 0.568 ** |
| P value | < 0.001 | |
| HbA1c | Correlation Coefficient | 0.628 ** |
| P value | < 0.001 | |
| HOMA IR | Correlation Coefficient | 0.430** |
| P value | < 0.001 | |
| Insulin Level (mIU/mL) | Correlation Coefficient | 0.255** |
| P value | 0.001 | |
| ** Correlation is significant at the 0.01 level (2-tailed) | ||
4. DISCUSSION
Netrin-1 is a member of the laminin-related proteins of axon guidance and has been shown to have a variety of roles via its two traditional receptor families, including deleted receptors in colorectal cancer (DCC) and uncoordinated 5 (UNC5).15 Netrin-1, which functions as a neuro-immune signaling molecule, has a significant impact on embryonic pancreas development. Regeneration, tissue remodeling, islet cell migration, and pancreatic morphogenesis are all impacted by netrin-1.16,17
This study found that individuals with T2DM had significantly higher mean serum netrin-1 values compared to controls, which is likely due to a compensatory reaction to the disease. Several other studies revealed similar finding.18–22

The current study revealed that there was significant positive correlation between patients age and serum netrin-1 level (r = 0.054, P = 0.497). Also, the patients mean value of serum netrin-1 levels were greater than those of the control group at the same age group, with a highly significant difference (P < 0.001). These results were consistent with those of other studies.23,24,25
Also, there was a significant positive relationship between serum netrin-1 levels and HbA1c. Similar finding also stated by other studies.18–22 In contrary some others studies reported controversial result.26, 27,28
This variation in relation to the association between T2DM and serum netrin-1 is indistinct, which could be imputed to diversity with respect to specimen collection, sample size, ethnic group, other sociodemographic differences of the investigated population, and estimation method. Additionally, the result of this study showed a significant positive association between serum glucose levels and HbA1c with serum netrin-1 levels (r = 0.568, P < 0.001; r = 0.628, P = 0.001 respectively). These findings were in concordance with the results of other studies.8,21,29,30,31 However, some other studies demonstrated a controversial result.25,26,28 Gao et al.3 stated that netrin-1 stimulates insulin release from the pancreas by accelerating calcium inflow to the β-cell and the generation of the cAMP. The observation of this study may provide an insight that serum netrin-1 level may be used as an indicator for the control of T2DM.
The HOMA-IR is a measurement that assesses insulin resistance levels in the body and high value of HOMA-IR refers to the decreased ability of cells to respond properly to insulin and glucose load. The development of diabetes and other metabolic disorders can be delayed with an early diagnosis of insulin resistance. The success of diabetes treatment can also be tracked by clinicians using the HOMA, who can then modify the treatment strategy as necessary.6 The study revealed a positive correlation between serum netrin-1and HOMA-IR. This result was in agreement with finding of Natura G, who stated that netrin-1 could coordinate inflammation, which might lead to negative regulation of insulin secretion from β-cell and participate into β-cell failure.32 Several studies revealed similar findings.8,30,33,34,35 Ramkhelawon et al.34 stated that insulin resistance perturbs the netrin-1 circulation and netrin-1 over expression supports imperfection of adipose tissue immigration and macrophage retention, which might possibly enhance the promotion of chronic inflammation, metabolic dysfunction and insulin resistance. However, other studies revealed controversial results.26 They stated that netrin-1 has a retention cue for macrophage in visceral adipose tissue which possibly enhance the progression of the chronic inflammation and the subsequent development of insulin resistance, that happens in T2DM.26 It has been concluded that netrin-1 may have a regulatory effect on inflammatory process which might lead to negative regulation of insulin secretion and may have a role in dysfunction of β-cell.32
5. CONCLUSION
In conclusion the contradictory results with respect to the association between netrin-1 and type 2 diabetes mellitus, and the association between netrin-1 and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) may require further studies involving a larger sample size to clarify the real relationship and to improve reliability and replicability and to provide an insight to the pathogenesis, diagnosis, prevention, and treatment of type 2 diabetes mellitus.
6. Data availability
The numerical data generated during this research is available with the authors.
7. Acknowledgement
We gratefully thank Department of Biochemistry, College of Medicine, University of Basrah, for their generous help in completion of this research.
8. Conflict of interest
The study utilized the hospital resources only, and no external or industry funding was involved.
9. Authors’ contribution
AAA: Drafting the manuscript
HAK, NAN: Conduction of the study work and manuscript editing
MMMA: Evaluation and sending the manuscript
10. REFERENCES
- Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269-73. PMID: 23071876 PMCID: PMC3464757 DOI: 5001/omj.2012.68
- Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: worldwide difference of prevalence and risk factors. Journal of nephropharmacology. 2016;5(1):49. PMID: 28197499 PMCID: PMC5297507
- Gao S, Zhang X, Qin Y, Xu S, Zhang J, et al. Dual actions of Netrin-1 on islet insulin secretion and immune modulation. Clin sci (Lond). 2016;130(21):1901-11. PMID: 27520508; DOI: 1042/CS20160133
- Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. Insulin signalling in the heart. Cardiovascular research. 2008;79(2):238-48. PMID: 18390897 DOI: 1093/cvr/cvn093
- Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978;27(12):1893-902. PMID: 723640 DOI: 1016/s0026-0495(78)80007-9
- Xing Y, Lai J, Liu X, Zhang N, Ming J, Liu H, Zhang X. Netrin-1 restores cell injury and impaired angiogenesis in vascular endothelial cells upon high glucose by PI3K/AKT-eNOS. Journal of molecular endocrinology. 2017;58(4):167-77. PMID: 28250059; DOI: 1530/JME-16-0239
- Hacıhamdioğlu DÖ, Hacıhamdioğlu B, Altun D, Müftüoğlu T, Karademir F, Süleymanoğlu S. Urinary netrin-1: a new biomarker for the early diagnosis of renal damage in obese children. J Clin Res Pediatr Endocrinol. 2016 Sep 1;8(3):282-7. PMID: 27087488 PMCID: PMC5096491 DOI: 4274/jcrpe.2828
- Yim J, Kim G, Lee BW, Kang ES, Cha BS, Kim JH, Cho JW, Lee SG, Lee YH. Relationship between circulating netrin-1 concentration, impaired fasting glucose, and newly diagnosed type 2 diabetes. Front Endocrinol. 2018;9:691. PMID: 30532735 PMCID: PMC6265472 DOI: 3389/fendo.2018.00691
- De Breuck S, Lardon J, Rooman I, Bouwens L. Netrin-1 expression in fetal and regenerating rat pancreas and its effect on the migration of human pancreatic duct and porcine islet precursor cells. Diabetologia.2003;46:926-33. PMID: 12819897 DOI: 1007/s00125-003-1125-5
- Yang YH, Szabat M, Bragagnini C, Kott K, Helgason CD, Hoffman BG, Johnson JD. Paracrine signalling loops in adult human and mouse pancreatic islets: netrins modulate beta cell apoptosis signalling via dependence receptors. Diabetologia. 2011;54:828-42. PMID: 21212933 DOI: 1007/s00125-010-2012-5
- Bloomgarden ZT. Inflammation and insulin resistance. Diabetes care. 2003;26(6):1922-6. PMID: 12766135 DOI: 2337/diacare.26.6.1922
- Kang YM, Kim F, Lee WJ. Role of NO/VASP signaling pathway against obesity-related inflammation and insulin resistance. Diabetes Metab J. 2017;41(2):89-95. PMID: 28447436 PMCID: PMC5409001 DOI: 4093/dmj.2017.41.2.89
- Gillett MJ. International expert committee report on the role of the A1c assay in the diagnosis of diabetes: diabetes care 2009;32(7): 1327–1334. Clin Biochem Rev. 2009 Nov;30(4):197-200. PMID: 20011212 PMCID: PMC2791773
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. diabetologia.1985;28:412-9. PMID: 3899825 DOI: 1007/BF00280883
- Serafini T, Kennedy TE, Gaiko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78(3):409-24. PMID: 8062384 DOI: 1016/0092-8674(94)90420-0
- Jayakumar C, Mohamed R, Ranganathan PV, Ramesh G. Intracellular kinases mediate increased translation and secretion of netrin-1 from renal tubular epithelial cells. PLoS One. 2011;6(10):e26776. PMID: 22046354 PMCID: PMC3202578 DOI: 1371/journal.pone.0026776
- Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia–reperfusion injury. J Immunol. 2010;185(6):3750-8. PMID: 20693423 DOI: 4049/jimmunol.1000435
- Garcia Galindo JJ, Ramos-Zavala MG, Pascoe-Gonzalez S, Hernández-González SO, Delgadillo-Centeno JS, Grover-Páez F, et al. Association of Netrin 1 with hsCRP in Subjects with Obesity and Recent Diagnosis of Type 2 Diabetes. Curr Issues Mol Biol. 2022 Dec 26;45(1):134-140. PMID: 36661496 PMCID: PMC9857863 DOI: 3390/cimb45010010
- Guo D, Zhu Z, Zhong C, Peng H, Wang A, Xu T, Peng Y, Xu T, Chen CS, Li Q, Ju Z. Increased serum netrin-1 Is associated with improved prognosis of ischemic stroke: an observational study from CATIS. Stroke. 2019;50(4):845-52. PMID: 30852966 DOI: 1161/STROKEAHA.118.024631
- Sadeq AB, Al-Saeed HH, Rasheed AM. Study on the Levels of Serum Netrin-1(NTN-1) in Type-2 Diabetic Patients with And without Retinopathy. Biochemical and Cellular Archives21(1):457-462
- Selim A, El Hai DA, Lashin F, Salem HH. The Role of Netrin-1 and Interleukin-6 in Diabetic Nephropathy in Patients with Type 2 Diabetes Mellitus. Journal of Advances in Medicine and Medical Research.2022; 34(22)::61-68.DOI: 9734/jammr/2022/v34i2231579
- Rosaria E, Kurniawan LB. Correlation of Glycated Haemoglobin with Netrin-1 and High Sensitive C-Reactive Proteinin Type 2 Diabetes Melitus Patients. Medico-Legal Update. 2020;20(4):1113-1118. DOI: https://doi.org/10.37506/mlu.v20i4.1976
- Yimer EM, Zewdie KA, Hishe HZ. Netrin as a novel biomarker and its therapeutic implications in diabetes mellitus and diabetes-associated complications. Diabetes Res. 2018 Sep 18;2018:8250521. PMID: 30320139 PMCID: PMC6167572 DOI: 1155/2018/8250521
- Fadel MM, Ghaffar FR, Zwain SK, Ibrahim HM. Serum netrin and VCAM-1 as biomarker for Egyptian patients with type IΙ diabetes mellitus. Biochem Biophys Rep. 2021 Jun 11;27:101045 PMID: 34179515 PMCID: PMC8209750 DOI: 1016/j.bbrep.2021.101045
- Inderjeet K, Adole PS, Vinod KV, Pillai AA. Association between serum netrin-1, netrin-4 and risk of the acute coronary syndrome in patients with type 2 diabetes mellitus-A pilot study. Indian Heart J. 2022;74(1):72-5 PMID: 34875255 PMCID: PMC8891007 DOI: 1016/j.ihj.2021.11.186
- Liu C, Ke X, Wang Y, Feng X, Li Q, Zhang Y, et al. The level of netrin-1 is decreased in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr Disord. 2016;16(1):33. PMID: 27255377 PMCID: PMC4890509 DOI: 1186/s12902-016-0112-z
- Assi MA, Hammood HJ, Attia ZM. Relationship between Netrin-1 Levels and Diabetes Mellitus Type 2. NeuroQuantology. 2021;19(10):29.-33 DOI: 14704/nq.2021.19.10.NQ21153
- ACAR ÇA, PEHLİVANOĞLU S, Mehmet KÖ, YILMAZ G. Evaluation of serum levels of Netrin-1 as a potential biomarker for early prediction of prediabetes. Turkish Journal of Health Science and Life. 2021;4(2):70-6. [Free article]
- Ay E, Marakoğlu K, Kizmaz M, Ünlü A. Evaluation of Netrin‐1 levels and albuminuria in patients with diabetes. J Clin Lab Anal. 2016 Nov;30(6):972-977 PMID: 27076403 PMCID: PMC6806737 DOI: 1002/jcla.21965
- Khalil MM, Ali HA. Evaluation of Serum Netrin-1 level as a Diagnostic Biomarker in Patients with Diabetes. Biochemical & Cellular Archives. 2021;21(2): 4953-4957. [Free article]
- Usha A, Sriprajna M, Sudindra R, Menambath DT. Netrin-1 and Insulin Resistance as markers in Type 2 Diabetes Mellitus. Journal of Livestock Science (ISSN online 2277-6214).;14:148-54.DOI: 33259/JLivestSci.2023.148-154
- Natura G, Bär KJ, Eitner A, Boettger MK, Richter F, Hensellek S, et al. Neuronal prostaglandin E2 receptor subtype EP3 mediates antinociception during inflammation. Proc Natl Acad Sci U S A.;110(33):13648-53. PMID: 23904482 PMCID: PMC3746931 DOI: 1073/pnas.1300820110
- Ranganathan PV, Jayakumar C, Ramesh G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. American Journal of Physiology-Renal Physiology.2013;304(7): F948-57.
- PMID: 23408164 PMCID: PMC3625850 DOI: 1152/ajprenal.00580.2012
- Ramkhelawon B, Hennessy EJ, Ménager M, Ray TD, Sheedy FJ, Hutchison S, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20(4):377-84. PMID: 24584118 PMCID: PMC3981930 DOI: 1038/nm.3467
- Jung HI, Bae J, Han E, Kim G, Lee JY, Kim SR, et al. Circulating Netrin-1 as a novel biomarker for impaired fasting glucose and newly diagnosed type 2 diabetes mellitus. Diabetes.2018;67(Supplement_1). DOI: 2337/db18-1535-P