Samuel Hotma Rotua , Dwisetyo Gusti Arilaksono , Shinta Vera Hutajulu
Author affiliation:
Pertamina Jaya Hospital, Jl. Jend. Ahmad Yani No.2, RT.2RW.7, Cemp. Putih Tim., Kec. Cemp. Putih, Kota Jakarta Pusat, Daerah Khusus Ibukota Jakarta 10510, Indonesia.
Correspondence: Samuel Hotma Rotua Sinaga; E-mail: hotma.rotua89@gmail.com; Phone: 62214211911: Mobile: 62 8161637569
Abstract
COVID-19 has a wide spectrum of clinical presentations, from asymptomatic cases to severe respiratory distress, multi-organ dysfunction, and death. Pulmonary embolism (PE) is the most feared and severe complication of venous thromboembolism (VTE). We present a case of a 25-year-old nonsmoker pregnant woman (gravida 2, no history of abortion/miscarriage), who underwent an elective cesarean section at 39 weeks of gestation after uneventful pregnancy. She exhibit clinical presentation of pulmonary embolism (PE) overlapping with severe COVID-19 pneumonia. The diagnosis was made based upon severe oxygen desaturation, McConnel sign finding on POCUS and elevated D-dimer level (34.19 µg/mL). Alteplase and low-molecular-weight heparin were used, which resulted in rapid clinical improvement. We should be warned about high or extremely elevated D-dimer levels and severe oxygen desaturation, as markers of severe COVID-19 pneumonia in patients with high clinical suspicion of PE. Thrombolysis could be an effective and safe therapy for PE in ARDS secondary to COVID-19. Furthermore, we underline that POCUS, despite its inherent limitations, could be a flexible diagnostic and management tool in refractory ARDS due to COVID-19.
Key words: COVID-19; SARS-CoV-2; Pulmonary Embolism; Thrombolysis; Postpartum; POCUS
Abbreviations: ARDS - Acute respiratory distress syndrome; PE - Pulmonary embolism; VTE - Venous thromboembolism; BPM – Beats per minute
Citation: Rotua SH, Arilaksono DG, Hutajulu SV. Systemic thrombolysis and anticoagulation in postpartum patient with acute respiratory distress syndrome, COVID-19 and acute pulmonary embolism: a case report. Anaesth. pain intensive care 2022;26(1):115-118. DOI: 10.35975/apic.v26i1.1777
Received: September 13, 2021, Reviewed: November 06, 2021, Accepted: November 10, 2021
Introduction
Coronavirus has diverse clinical presentations from asymptomatic cases to respiratory distress from mild to severe, multi-organ dysfunction, and sometimes death. The deadly COVID-19 clinical presentation is characterized by ARDS, sepsis, thromboembolic disease, and multi-system organ failure.1 The thromboembolic disease integrates the phenomena of both venous and arterial thromboembolism; while the underlying pathophysiology remains poorly understood. Pulmonary embolism (PE) is the most severe variety of the venous thromboembolism (VTE). Despite the fact that almost all patients got at least prophylactic doses of anticoagulant therapy, incidence of PE in critically ill COVID-19 patients in the Intensive Care Unit (ICU) has been 11.1%.2 Before the COVID-19 era, pregnancy-associated PE was not common, but remained potentially fatal and one of the leading causes of maternal mortality and morbidity in many countries, especially in the developed ones.3 We present the case of a 25-years-old woman with acute pulmonary embolism and COVID-19, 14 days after a scheduled cesarean section.
Case Report
A healthy, non-smoker, 25-years-old woman (gravida 2, 1 full term infant delivered, no abortions / miscarriages), underwent an elective cesarean section at 39 weeks of gestation after an uncomplicated pregnancy. There were no complications with the delivery, and both the mother and the child were healthy and discharged from the hospital. On 10th postpartum day, she developed a cough, high fever, and mild shortness of breath. She later tested positive for COVID-19 and was hospitalized. She had no known history of contact with people with confirmed or suspected COVID-19 during her pregnancy and after being discharged from the hospital. On the third day, she developed worsening shortness of breath and was referred to our hospital.
Physical examination at admission revealed; blood pressure 121/81 mmHg, body temperature 37°C, pulse
rate 132 BPM, respiratory rate 30 BPM, and oxygen saturation 89% with 15 L/min oxygen through a non–rebreathing mask. Her body mass index was 24.4 kg/cm2, and physical examination was within the normal value. We exchanged non–rebreathing mask with high flow nasal cannula (HFNC), improving oxygen saturation to 94%, and pulse rate to 111 BPM.
A chest X-ray showed bilateral ground-glass opacities and pulmonary infiltrates (Figure 1). The ER department electrocardiogram (ECG) showed sinus tachycardia at 125 BPM. QTc was 577 ms (using Bazett’s formula). Blood tests showed leukocytosis, hypoalbuminemia and hypokalemia. D-dimer level was 34.19 µg/mL (< 0.5 µg/mL). Blood gas analysis showed respiratory alkalosis (Table 1). She used HFNC with a flow of 60 L/min and
90%. PaO2/FiO2 ratio was 181.1 and a diagnosis of acute respiratory distress syndrome (ARDS) with
COVID-19 was made, and she was transferred to the Intensive Care Unit (ICU).
In the ICU Point-of-Care-Ultrasound (POCUS) was performed and it was revealed that there was a severe right ventricular dysfunction, hypokinesia, left ventricular ejection fraction (LVEF) of 65% by the Teicholz method, tricuspid annular plane systolic excursion (TAPSE) 18 mm, mild tricuspid regurgitation (TR) and a positive McConnell Sign (Figure 2).
Based on the chest X-ray, blood tests, and POCUS findings, we diagnosed her with pulmonary embolism and decided to start treatment with alteplase 25 mg in 2 h, followed by 25 mg in 22 h and then LWMH 20.000 iu for 24 h. The patient also received remdesivir 200 mg OD, tab vitamin C 3 x 1 G, n-acetylcysteine 5 G, vitamin D3 10000 iu, zinc 60 mg, atorvastatin 40 mg, as well as two bags of convalescent plasma for COVID-19.
The patient made an uneventful recovery without any bleeding complications. Gradually she could be weaned off the HFNC to non–rebreathing mask. By the 10th day she was discharged from the ICU.
Follow-up POCUS on 14th day of hospitalization showed no right heart thrombi, no McConnell sign, and restored right ventricular function (Figure 3). LVEF was 69% with global normokinesia, and TAPSE 17mm with trivial to mild TR.
Chest X-ray showed improvement in pneumonia status (Figure 4). The RT-PCR for COVID-19 was negative on day 17. After 18 days of hospitalization, the patient was discharged on n-acetylcysteine 600 mg, vitamin C 1 G, and rivaroxaban 15 mg.
Discussion
COVID-19 refractory ARDS can be caused by the interaction of inflammatory pathologies which can affect both lung ventilation and perfusion. Since patients with severe COVID-19 disease have cardiopulmonary comorbidities and proinflammatory risk factors related to the inflammation, they have high risk of thrombosis. Ng et al. reported that the thromboembolic risk is related to COVID-19 infection and that a diagnosis of pulmonary embolism has to be taken into consideration in any patient with respiratory failure.2
Our patient had high levels of D-dimer (34.19 µg/mL), and high D-dimer levels have been reported to increased mortality rate and a high prevalence of thrombosis in COVID-19.4 COVID-19 resulting in a cytokine storm, which can induce activation of endothelial cell damage, arterial inflammation, escalated platelet aggregation, increased sensitivity of thrombocytes, as well as disruption of vulnerable plaques.5 Interleukin-6 (IL-6) causes abnormality of antithrombin III, protein S, and thrombomodulin, as well as the expression of numerous prothrombotic factors.5 All these mechanisms can induce extended microangiopathy with micro and macro thrombosis. Furthermore, COVID-19 may predispose patients to both venous and arterial thrombosis secondary to hypoxia, immobilization, antiphospholipid antibodies, and diffuse intravascular coagulation.6
During pregnancy, changes in coagulation pathways increases the risk of embolic events. The risk is particularly significant within the immediate postpartum period. Venous embolism is an important cause of maternal illness and death.3 Although half of all cases occur during pregnancy and the other half during the postpartum period, the risk per day is the highest in the weeks following delivery.3 There are plenty of studies supporting the notion that cesarean delivery is related to a doubled risk of thrombosis when compared to vaginal delivery (OR = 2).7
Some guidelines specifically pertain to thrombolysis during pregnancy and the postpartum period. Martillotti reviewed 83 cases of pregnancy-related pulmonary embolism that were treated with thrombolytic therapy.8 Alteplase was the most commonly used thrombolytic agent, with a dose and duration of 100 mg and 2 hours, although 16 women received doses below 100 mg (15.5 to 90 mg). Among all the cases, the postpartum PE survival rate was 84.2% (16/19; 95% CI, 60–97). The exploration of major bleeding risk with thrombolytic drugs was limited to a small number of events: the risk of bleeding was 20% for rtPA (62% postpartum) and 58% for streptokinase (50% postpartum).8
COVID-19 therapeutic strategies targeting the pulmonary circulation are likely to require a multimodal approach. Thrombolysis has previously been shown to remove vascular obstructions in ARDS patients. Abdelaal Ahmed reported that compared to the unfractionated heparin group, the streptokinase group had improved lung compliance, pCO2, and reduced ICU mortality.9 Manipulating the fibrinolytic system by administering of t-PA may have a role in the therapy of severe, medically refractory ARDS induced by COVID-19.
Echocardiography is usually recommended when high-risk PE is suspected, when the patient’s condition is so critical that only bedside diagnostic testing can be allowed. POCUS has become popular as an adjunctive tool for the surveillance of the development of VTE in patients with COVID-19.9
This case report demonstrates that the administration of enhanced anticoagulation to patients with severe COVID-19, and D-dimer > 3.0 µg/ml may be a necessary critical care practice.6 Clearly, the risk of bleeding cannot be underestimated; therefore, these patients require close monitoring in an ICU. No specific guidelines exist for the management of PE in COVID-19. Thrombolysis can be an effective and safe treatment for PE in ARDS + COVID-19 patients. POCUS can be a flexible tool for diagnostic and treatment purposes in the refractory ARDS due to COVID-19.10
Conflict of interests
None declared by the authors. No external funding was involved in the conduct of this case.
Authors’ contribution
SHR: Concept, conduction of the study work, manuscript writing
DAG, SVH: Manuscript editing
References
Author affiliation:
Pertamina Jaya Hospital, Jl. Jend. Ahmad Yani No.2, RT.2RW.7, Cemp. Putih Tim., Kec. Cemp. Putih, Kota Jakarta Pusat, Daerah Khusus Ibukota Jakarta 10510, Indonesia.
Correspondence: Samuel Hotma Rotua Sinaga; E-mail: hotma.rotua89@gmail.com; Phone: 62214211911: Mobile: 62 8161637569
Abstract
COVID-19 has a wide spectrum of clinical presentations, from asymptomatic cases to severe respiratory distress, multi-organ dysfunction, and death. Pulmonary embolism (PE) is the most feared and severe complication of venous thromboembolism (VTE). We present a case of a 25-year-old nonsmoker pregnant woman (gravida 2, no history of abortion/miscarriage), who underwent an elective cesarean section at 39 weeks of gestation after uneventful pregnancy. She exhibit clinical presentation of pulmonary embolism (PE) overlapping with severe COVID-19 pneumonia. The diagnosis was made based upon severe oxygen desaturation, McConnel sign finding on POCUS and elevated D-dimer level (34.19 µg/mL). Alteplase and low-molecular-weight heparin were used, which resulted in rapid clinical improvement. We should be warned about high or extremely elevated D-dimer levels and severe oxygen desaturation, as markers of severe COVID-19 pneumonia in patients with high clinical suspicion of PE. Thrombolysis could be an effective and safe therapy for PE in ARDS secondary to COVID-19. Furthermore, we underline that POCUS, despite its inherent limitations, could be a flexible diagnostic and management tool in refractory ARDS due to COVID-19.
Key words: COVID-19; SARS-CoV-2; Pulmonary Embolism; Thrombolysis; Postpartum; POCUS
Abbreviations: ARDS - Acute respiratory distress syndrome; PE - Pulmonary embolism; VTE - Venous thromboembolism; BPM – Beats per minute
Citation: Rotua SH, Arilaksono DG, Hutajulu SV. Systemic thrombolysis and anticoagulation in postpartum patient with acute respiratory distress syndrome, COVID-19 and acute pulmonary embolism: a case report. Anaesth. pain intensive care 2022;26(1):115-118. DOI: 10.35975/apic.v26i1.1777
Received: September 13, 2021, Reviewed: November 06, 2021, Accepted: November 10, 2021
Introduction
Coronavirus has diverse clinical presentations from asymptomatic cases to respiratory distress from mild to severe, multi-organ dysfunction, and sometimes death. The deadly COVID-19 clinical presentation is characterized by ARDS, sepsis, thromboembolic disease, and multi-system organ failure.1 The thromboembolic disease integrates the phenomena of both venous and arterial thromboembolism; while the underlying pathophysiology remains poorly understood. Pulmonary embolism (PE) is the most severe variety of the venous thromboembolism (VTE). Despite the fact that almost all patients got at least prophylactic doses of anticoagulant therapy, incidence of PE in critically ill COVID-19 patients in the Intensive Care Unit (ICU) has been 11.1%.2 Before the COVID-19 era, pregnancy-associated PE was not common, but remained potentially fatal and one of the leading causes of maternal mortality and morbidity in many countries, especially in the developed ones.3 We present the case of a 25-years-old woman with acute pulmonary embolism and COVID-19, 14 days after a scheduled cesarean section.
Case Report
 Figure 1: Chest X-ray showing bilateral ground-glass opacities and pulmonary infiltrates |
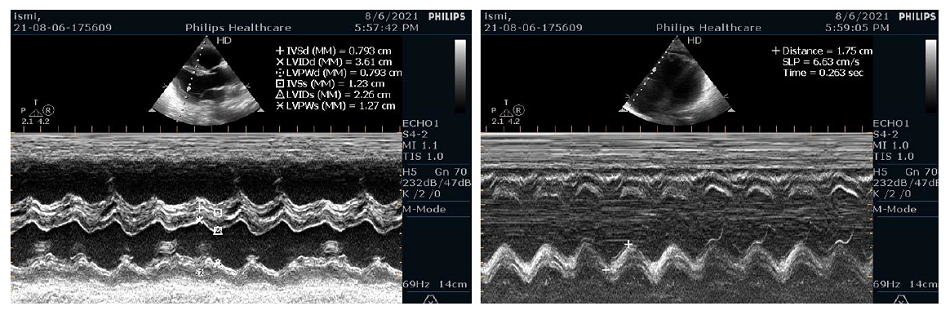 Figure 2: POCUS showed right ventricular dilatation |
| Table 1: Patient’s laboratory data (normal range) | |
| Indicator | Value (normal values) |
| Hemoglobin Leukocytes Lymphocytes Platelets Glucose Creatinine Albumin Sodium Potassium Chloride D-dimer pH pO2 pCO2 HCO3 BE O2 Saturation |
10.6 g/dL (12.0–14.0 g/dL) 13.9 x 103/µL (5.0–10.0 x 103/µL) 11.6 % (20.0– 40.0 %) 357 x 103/mL (150–450 x 103/mL) 105 mg/dL (70–120 mg/dL) 0.6 mg/dL (0.6-1.1 mg/dL) 3.2 g/dL (3.8–5.2 g/dL) 138 mEq/L (135–145 mEq/L) 3.1 mEq/L (3.5–5.1 mEq/L) 100 mEq/L (98–107 mEq/L) 34.19 µg/mL (< 0.5 µg/mL) 7.519 mmol/L (7.35 - 7.45 mmol/L) 163 mmHg (85 - 95 mmHg) 29 mmHg (35 - 45 mmHg) 23 mmol/L (21 - 25 mmol/L) 0.20 mmol/L (-2.50 - 2.50 mmol/L) 99 % (85 - 95 %) |
A chest X-ray showed bilateral ground-glass opacities and pulmonary infiltrates (Figure 1). The ER department electrocardiogram (ECG) showed sinus tachycardia at 125 BPM. QTc was 577 ms (using Bazett’s formula). Blood tests showed leukocytosis, hypoalbuminemia and hypokalemia. D-dimer level was 34.19 µg/mL (< 0.5 µg/mL). Blood gas analysis showed respiratory alkalosis (Table 1). She used HFNC with a flow of 60 L/min and
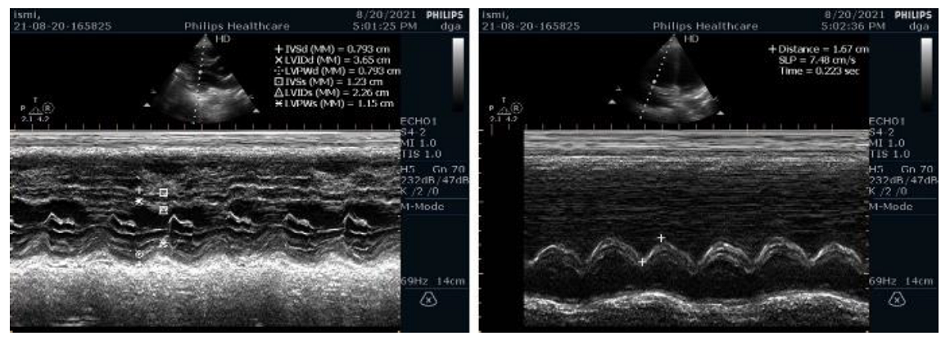 Figure 3: Follow Up POCUS showed restored right ventricular function |
COVID-19 was made, and she was transferred to the Intensive Care Unit (ICU).
In the ICU Point-of-Care-Ultrasound (POCUS) was performed and it was revealed that there was a severe right ventricular dysfunction, hypokinesia, left ventricular ejection fraction (LVEF) of 65% by the Teicholz method, tricuspid annular plane systolic excursion (TAPSE) 18 mm, mild tricuspid regurgitation (TR) and a positive McConnell Sign (Figure 2).
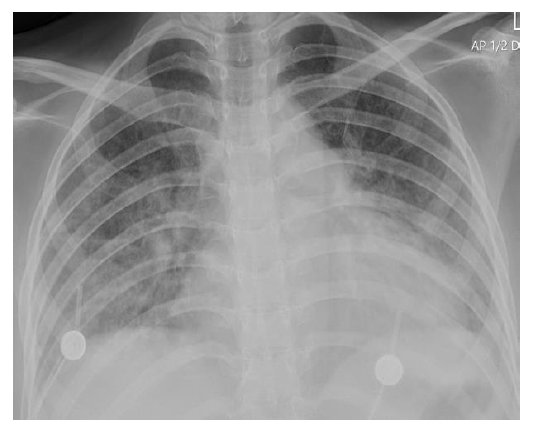 Figure 4: Chest X-ray shows marked improvement |
The patient made an uneventful recovery without any bleeding complications. Gradually she could be weaned off the HFNC to non–rebreathing mask. By the 10th day she was discharged from the ICU.
Follow-up POCUS on 14th day of hospitalization showed no right heart thrombi, no McConnell sign, and restored right ventricular function (Figure 3). LVEF was 69% with global normokinesia, and TAPSE 17mm with trivial to mild TR.
Chest X-ray showed improvement in pneumonia status (Figure 4). The RT-PCR for COVID-19 was negative on day 17. After 18 days of hospitalization, the patient was discharged on n-acetylcysteine 600 mg, vitamin C 1 G, and rivaroxaban 15 mg.
Discussion
COVID-19 refractory ARDS can be caused by the interaction of inflammatory pathologies which can affect both lung ventilation and perfusion. Since patients with severe COVID-19 disease have cardiopulmonary comorbidities and proinflammatory risk factors related to the inflammation, they have high risk of thrombosis. Ng et al. reported that the thromboembolic risk is related to COVID-19 infection and that a diagnosis of pulmonary embolism has to be taken into consideration in any patient with respiratory failure.2
Our patient had high levels of D-dimer (34.19 µg/mL), and high D-dimer levels have been reported to increased mortality rate and a high prevalence of thrombosis in COVID-19.4 COVID-19 resulting in a cytokine storm, which can induce activation of endothelial cell damage, arterial inflammation, escalated platelet aggregation, increased sensitivity of thrombocytes, as well as disruption of vulnerable plaques.5 Interleukin-6 (IL-6) causes abnormality of antithrombin III, protein S, and thrombomodulin, as well as the expression of numerous prothrombotic factors.5 All these mechanisms can induce extended microangiopathy with micro and macro thrombosis. Furthermore, COVID-19 may predispose patients to both venous and arterial thrombosis secondary to hypoxia, immobilization, antiphospholipid antibodies, and diffuse intravascular coagulation.6
During pregnancy, changes in coagulation pathways increases the risk of embolic events. The risk is particularly significant within the immediate postpartum period. Venous embolism is an important cause of maternal illness and death.3 Although half of all cases occur during pregnancy and the other half during the postpartum period, the risk per day is the highest in the weeks following delivery.3 There are plenty of studies supporting the notion that cesarean delivery is related to a doubled risk of thrombosis when compared to vaginal delivery (OR = 2).7
Some guidelines specifically pertain to thrombolysis during pregnancy and the postpartum period. Martillotti reviewed 83 cases of pregnancy-related pulmonary embolism that were treated with thrombolytic therapy.8 Alteplase was the most commonly used thrombolytic agent, with a dose and duration of 100 mg and 2 hours, although 16 women received doses below 100 mg (15.5 to 90 mg). Among all the cases, the postpartum PE survival rate was 84.2% (16/19; 95% CI, 60–97). The exploration of major bleeding risk with thrombolytic drugs was limited to a small number of events: the risk of bleeding was 20% for rtPA (62% postpartum) and 58% for streptokinase (50% postpartum).8
COVID-19 therapeutic strategies targeting the pulmonary circulation are likely to require a multimodal approach. Thrombolysis has previously been shown to remove vascular obstructions in ARDS patients. Abdelaal Ahmed reported that compared to the unfractionated heparin group, the streptokinase group had improved lung compliance, pCO2, and reduced ICU mortality.9 Manipulating the fibrinolytic system by administering of t-PA may have a role in the therapy of severe, medically refractory ARDS induced by COVID-19.
Echocardiography is usually recommended when high-risk PE is suspected, when the patient’s condition is so critical that only bedside diagnostic testing can be allowed. POCUS has become popular as an adjunctive tool for the surveillance of the development of VTE in patients with COVID-19.9
This case report demonstrates that the administration of enhanced anticoagulation to patients with severe COVID-19, and D-dimer > 3.0 µg/ml may be a necessary critical care practice.6 Clearly, the risk of bleeding cannot be underestimated; therefore, these patients require close monitoring in an ICU. No specific guidelines exist for the management of PE in COVID-19. Thrombolysis can be an effective and safe treatment for PE in ARDS + COVID-19 patients. POCUS can be a flexible tool for diagnostic and treatment purposes in the refractory ARDS due to COVID-19.10
Conflict of interests
None declared by the authors. No external funding was involved in the conduct of this case.
Authors’ contribution
SHR: Concept, conduction of the study work, manuscript writing
DAG, SVH: Manuscript editing
References
- Faqihi F, Alharthy A, Alodat M, Kutsogiannis DJ, Brindley PG, Karakitsos D. Therapeutic plasma exchange in adult critically ill patients with life-threatening SARS-CoV-2 disease: A pilot study. J Crit Care. 2020 Dec;60:328-333. [PubMed] DOI: 1016/j.jcrc.2020.07.001
- Ng JJ, Liang ZC, Choong AMTL. The incidence of pulmonary thromboembolism in COVID-19 patients admitted to the intensive care unit: a meta-analysis and meta-regression of observational studies. J Intensive Care. 2021 Feb 22;9(1):20. [PubMed] DOI: 1186/s40560-021-00535-x
- Chang J, Elam-Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA, Syverson CJ. Pregnancy-related mortality surveillance--United States, 1991--1999. MMWR Surveill Summ. 2003 Feb 21;52(2):1-8. [PubMed]
- Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020 May 14;41(19):1858. [PubMed] DOI: 1093/eurheartj/ehaa254
- Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA, Bohula EA. Covid-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020 Apr 10;5(5):518-536. [PubMed] DOI: 1016/j.jacbts.2020.04.002
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Jul;191:145-147. [PubMed] DOI: 1016/j.thromres.2020.04.013
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006 May;194(5):1311-5. [PubMed] DOI: 1016/j.ajog.2005.11.008
- Martillotti G, Boehlen F, Robert-Ebadi H, Jastrow N, Righini M, Blondon M. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemost. 2017 Oct;15(10):1942-1950. [PubMed] DOI: 1111/jth.13802
- Alharthy A, Faqihi F, Abuhamdah M, Noor A, Naseem N, Balhamar A, et al. Prospective longitudinal evaluation of point-of-care lung ultrasound in critically ill patients with severe covid-19 pneumonia. J Ultrasound Med. 2021 Mar;40(3):443-456. [PubMed] DOI: 10.1002/jum.15417