Daniel W. Kim, BS, BA1 , Amanda Hu, MD2 , Douglas Vaughn, MD, DDS1 , Govind RC Rajan, MBBS, FASA1
Author affiliations:
Abstract
Anesthetic management of patients with mitochondrial disorders is challenging due to their rare nature, and difficulties to develop evidence–based protocols for general anesthesia. We report a case of a 52–year–old male with hypertension, congenital deafness, optic neuritis, hypogonadism and myotonia, who developed severe refractory hypotension and bradycardia, and intractable severe lactic acidosis during induction of general anesthesia with fentanyl, propofol, and succinylcholine. The surgery had to be postponed and supportive management started. After extensive work–up, the patient was diagnosed with probable mitochondrial dysfunction. Patient returned for surgery under a different anesthetic regimen consisting of ketamine, fentanyl, ephedrine and dexmedetomidine; yielding a successful perioperative course and highlighting potential alternative strategies to manage anesthesia in patients with such disorders.
Key words: Mitochondrial Diseases; Propofol Infusion Syndrome; Lactic Acidosis
Abbreviations: MD – Mitochondrial disorders; PRIS – Propofol related infusion syndrome; HIPAA – Health Insurance Portability Accountability Act; MAP Mean arterial pressure; NMBAs – Neuromuscular blocking agents.
Citation: Kim DW, Hu A, Vaughn D, Rajan GRE. Hemodynamic collapse and severe metabolic acidosis following propofol in the setting of undiagnosed mitochondrial dysfunction: a case report. Anaesth. pain intensive care 2021;25(4):527–531.
DOI: 10.35975/apic.v25i4.1587
Received: September 24, 2020. Reviewed: April 1, 2021, Accepted: May 31, 2021
Introduction
Mitochondrial disorders (MD) are characterized by dysfunction of the oxidative phosphorylation process in the mitochondria, which involves variable modes of inheritance and variable ages at presentation. They have a broad range of effects on nearly every organ system including the heart, brain, skeletal muscle, and nervous system.1 Symptoms evolve over time and can present as developmental delay, encephalopathy, ataxia, optic neuritis, sensorineural deafness, dementia, retinopathy, diabetes mellitus, and/or liver failure.2,3
Since MD is extremely difficult to diagnose, formulating a specific anesthetic approach for these patients is challenging.4 Anesthetic agents primarily act on tissues with high–energy requirements that are profoundly dependent on mitochondrial function. Consequently, these patients are at increased risk of adverse events under general anesthesia.4,5,6 Propofol infusions, in particular, are associated with increased levels of serum acylcarnitines, which increase susceptibility to adverse reactions, such as propofol related infusion syndrome (PRIS). 4,5,6 We describe the development of intractable hypotension and severe lactic acidosis in a patient with undiagnosed mitochondrial dysfunction, following exposure to anesthetic agents, who underwent removal of bilateral ear prosthetic implant magnets. A written Health Insurance Portability Accountability Act (HIPAA) authorization and consent to use/disclose existing protected health information was obtained.
Case report
A 52–year–old male with hypertension, hyperlipidemia, congenital deafness, optic neuritis, hypogonadism, myotonia, gastroesophageal reflux disease, and hypothyroidism was scheduled for removal of bilateral ear prosthetic implant magnets under total intravenous anesthesia, as patient was construed to be high–risk for nausea and vomiting.
Pre–operative physical examination and laboratory work–up were unremarkable. Patient was administered midazolam 2 mg intravenously (IV) preoperatively and attached with routine monitors as per ASA guidelines. General anesthesia was induced with inj fentanyl 100 µg, propofol 200 mg (Fresenius Kabi, Sweden) and succinylcholine 60 mg. After smooth induction and intubation, anesthesia was maintained with propofol infusion at 50 µg/min and remifentanil at 0.2 µg/min. After surgical site preparation and time–out, empiric IV cefazolin 2 g was administered. Within a few minutes, patient became severely hypotensive (52/24 mmHg) and bradycardic (46 beats/min). Immediately, glycopyrrolate 0.3 mg, ephedrine 10 mg, and phenylephrine 200 µg were administered, but patient exhibited persistent hypotension and bradycardia. Epinephrine 0.3 mg IV bolus was administered, but to little effect. At this time, surgical procedure was aborted, and anesthetic infusions were discontinued. Patient was placed on an infusion of epinephrine at 3 µg/min and was titrated to maintain a mean arterial blood pressure (MAP) of 60 mmHg. Patient continued to experience severe refractory hypotension to epinephrine infusion and developed bigeminy, ST depression in lateral leads, and prolongation of PR interval. A radial arterial line and an additional intravenous line were secured. Anaphylaxis to medications administered, including cefazolin, was one possibility that came to our minds, so the patient was administered diphenhydramine 50 mg IV, dexamethasone 12 mg and one liter of normal saline. Epinephrine infusion was titrated up to 10 µg/min to maintain MAP of 60 mmHg. A 12–lead electrocardiogram (ECG) showed sinus tachycardia (90 beats/min) and ST depression in lateral leads. Of note, a rash was noticed near IV site along patient’s arm.
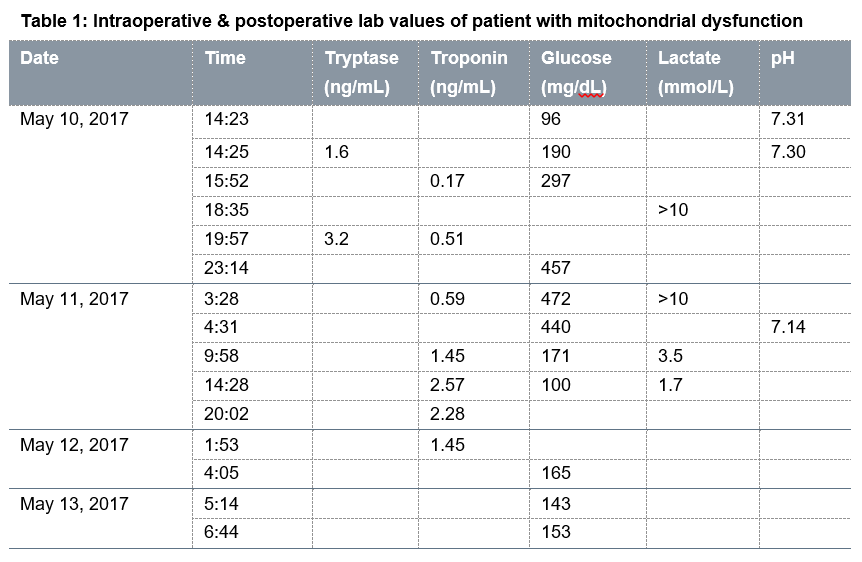
After discontinuation of propofol and remifentanil infusions and starting epinephrine infusion, patient’s condition gradually stabilized within 20 min. He was then successfully extubated. While breathing spontaneously on supplemental oxygen, he was transported from the outpatient surgery center to the main hospital post anesthesia care unit (PACU). Perioperative laboratory work–up yielded persistent, severe intractable lactic acidosis (>10 ng/mL; normal results range from 4.5 to 19.8 mg/dL), initially elevated troponin levels, normal tryptase levels, and a glucose spike that gradually decreased (Table 1).
 .
.
All subsequent ECG traces were within normal range with complete resolution of ST changes. Cardiology was consulted, and patient was transferred to cardiac care unit for close hemodynamic monitoring and further management. Patient was weaned off of epinephrine infusion over the next 24 hours. The lactic acidosis normalized over the next 48 hours. Patient had normal echocardiogram and negative Lexi scan nuclear stress test. Patient's allergy workup revealed an uneventful cefazolin challenge. Patient declined skin testing to propofol, succinylcholine, and neuromuscular blocking agents (NMBAs). During genetic evaluation, patient reported his mother passed away in her 50s after a relatively low–risk surgical procedure under general anesthesia. The patient refused muscle biopsy as part of MD workup. Patient returned three weeks later for the original surgery. A multidisciplinary team of physicians concluded that the patient suffered atypical propofol infusion syndrome in the face of undiagnosed MD and prescribed an anesthetic plan consisting of ketamine, dexmedetomidine and fentanyl. The patient tolerated that anesthetic and procedure very well, with no adverse events, and was discharged home the same day.
Discussion
MD appears to be the most plausible cause of this perioperative episode of hypotension, bradycardia, and intractable lactic acidosis after propofol administration. The patient’s medical and family history, along with negative allergy workup for the antibiotic used, leave little doubt that the propofol precipitated this clinical presentation. Patient’s history of muscle pain, fibromyalgia, diabetes, congenital deafness, and optic neuritis are consistent with the typical presentation of mitochondrial myopathy.2,3, In particular, because MD is maternally inherited, his mother’s unexpected death from a low–risk surgery under anesthesia raises suspicion of a mitochondrial–linked condition. After the patient was referred for genetic workup and counseling, the final diagnosis suggested was MD. However, patient refused a confirmatory muscle biopsy in order to avoid a similar reaction. Propofol is a mitochondrion–toxin thought to cause acute refractory bradycardia and metabolic acidosis in patients with mitochondrial myopathies.7, 8 Following propofol administration, the patient experienced severe type–B lactic acidosis, hypotension and bradycardia. Finally, patient returned for surgery and had an uneventful postoperative course under a different anesthetic regimen consisting of ketamine, fentanyl and dexmedetomidine, but avoiding propofol.
MD most commonly affects tissues highly dependent on oxidative metabolism, such as the nervous and muscular system.9 Pre-diagnosing and treating MD is a major challenge due to a highly variable clinical spectrum combined with genetic complexity associated with over 120 different mitochondrial DNA deletion types.4,9 Multiple genetic tests, muscle biopsies or brain scans may be required to confirm MD, but many subtypes can still go undiagnosed.4,10 However, surgery and anesthesia expose patients to triggers that can unmask underlying MDs.7 Because general anesthetics have their foremost clinical effect on highly oxygen dependent tissues, patients with MD may have an abnormal sensitivity to intravenous or volatile anesthetics. This may be particularly true with increasing age since bioenergetics appear to progressively depress from adulthood through senescence.9,11 Furthermore, intravenous anesthetic drugs depress properties such as oxygen consumption, energy production, and carbohydrate metabolism in the nervous system of MD patients. Local anesthetics also depress bioenergetics capacity and disrupt oxidative phosphorylation similarly to the mechanism in other intravenous agents.10
Propofol is mitochondrion–toxic, and therefore not safe in high dosages over prolonged periods of time in MD patients.7,12 Propofol markedly decreases oxygen consumption and ATP production in brain synaptosomes, reduces electron flow in cardiac mitochondria, and uncouples electron transport from ATP production.10 A rare complication of propofol is PRIS, which is characterized by acute refractory bradycardia, metabolic acidosis, rhabdomyolysis, myocardial failure, and even death.8,13. One predisposing factor for PRIS is subclinical MD. The physiopathology of PRIS remains unclear, but in animal studies, propofol disrupted electron flow along the respiratory chain and decreased multiple complexes.13 While PRIS occurs more often under long–term propofol infusion (> 48 h) at doses above 4 mg/kg/h, PRIS can be observed with lower doses and shorter duration of sedation.8 Therefore, propofol administration may be problematic in patients with MD.12 Lactic acid is derived from glucose metabolism via the glycolytic pathway and is primarily utilized in the liver and cleared by the kidney. Plasma lactate concentration exceeding 4 mmol/L defines lactic acidosis, categorized into two types.14 Type–A lactic acidosis is caused by impaired tissue oxygenation and hypoperfusion due to shock (septic, cardiogenic, hypovolemic, obstructive), regional ischemia, or seizures. Type–B lactic acidosis is rarer and unrelated to tissue hypoperfusion. Instead, it is due to alternative metabolic pathways for pyruvate, including liver disease, medications, diabetic ketoacidosis, trauma, and MD. This patient’s presentation illustrates type–B lactic acidosis due to MD.
To our knowledge, this is the first case of suspected atypical PRIS due to underlying MD unmasked by a low propofol infusion rate over a very short period of time (around 10 to 15 min). In MD patients, the safest anesthetic regimen remains unknown, but recommendations include avoiding anesthetics such as propofol and succinylcholine,4,5,6 minimally dosing volatiles or intravenous anesthetics, utilizing neuraxial or regional techniques, performing workup of MD (e.g. genetic tests, muscle biopsies), and thorough reviewing of previous anesthetics with patient and family. Alternatives may be considered, such as dexmedetomidine, a selective α2–adrenergic agonist with beneficial effects on mitochondrial membranes in ischemic rats and MD patients in some case reports.15
Conducting clinical trials and establishing evidence–based guidelines for anesthetic management of patients with MD is challenging due to difficulties in diagnosing this rare disease, rendering case reports as the most likely source of information. Much work still needs to be done to determine the safest and most efficacious guidelines for perioperative management of MD. This case report represents an important contribution to the growing body of evidence of anesthetic administration uncovering MDs and consequently formulating safe anesthetics for these patients.
Authors’ contributions
DWK, AH: Literature review, preparation of the manuscript
DV: Provision of anesthetic care, literature review, preparation of the manuscript
GR: Provision of anesthetic care, literature review, preparation of the manuscript, and guidance to primary authors
Conflict of Interest
None declared by the authors. No financial disclosure declared by any of the authors.
References
Author affiliations:
- University of California, Irvine, CA 92697, USA
- University of Washington, School of Medicine, 1959 NE Pacific St, Seattle, WA 98195, USA
Abstract
Anesthetic management of patients with mitochondrial disorders is challenging due to their rare nature, and difficulties to develop evidence–based protocols for general anesthesia. We report a case of a 52–year–old male with hypertension, congenital deafness, optic neuritis, hypogonadism and myotonia, who developed severe refractory hypotension and bradycardia, and intractable severe lactic acidosis during induction of general anesthesia with fentanyl, propofol, and succinylcholine. The surgery had to be postponed and supportive management started. After extensive work–up, the patient was diagnosed with probable mitochondrial dysfunction. Patient returned for surgery under a different anesthetic regimen consisting of ketamine, fentanyl, ephedrine and dexmedetomidine; yielding a successful perioperative course and highlighting potential alternative strategies to manage anesthesia in patients with such disorders.
Key words: Mitochondrial Diseases; Propofol Infusion Syndrome; Lactic Acidosis
Abbreviations: MD – Mitochondrial disorders; PRIS – Propofol related infusion syndrome; HIPAA – Health Insurance Portability Accountability Act; MAP Mean arterial pressure; NMBAs – Neuromuscular blocking agents.
Citation: Kim DW, Hu A, Vaughn D, Rajan GRE. Hemodynamic collapse and severe metabolic acidosis following propofol in the setting of undiagnosed mitochondrial dysfunction: a case report. Anaesth. pain intensive care 2021;25(4):527–531.
DOI: 10.35975/apic.v25i4.1587
Received: September 24, 2020. Reviewed: April 1, 2021, Accepted: May 31, 2021
Introduction
Mitochondrial disorders (MD) are characterized by dysfunction of the oxidative phosphorylation process in the mitochondria, which involves variable modes of inheritance and variable ages at presentation. They have a broad range of effects on nearly every organ system including the heart, brain, skeletal muscle, and nervous system.1 Symptoms evolve over time and can present as developmental delay, encephalopathy, ataxia, optic neuritis, sensorineural deafness, dementia, retinopathy, diabetes mellitus, and/or liver failure.2,3
Since MD is extremely difficult to diagnose, formulating a specific anesthetic approach for these patients is challenging.4 Anesthetic agents primarily act on tissues with high–energy requirements that are profoundly dependent on mitochondrial function. Consequently, these patients are at increased risk of adverse events under general anesthesia.4,5,6 Propofol infusions, in particular, are associated with increased levels of serum acylcarnitines, which increase susceptibility to adverse reactions, such as propofol related infusion syndrome (PRIS). 4,5,6 We describe the development of intractable hypotension and severe lactic acidosis in a patient with undiagnosed mitochondrial dysfunction, following exposure to anesthetic agents, who underwent removal of bilateral ear prosthetic implant magnets. A written Health Insurance Portability Accountability Act (HIPAA) authorization and consent to use/disclose existing protected health information was obtained.
Case report
A 52–year–old male with hypertension, hyperlipidemia, congenital deafness, optic neuritis, hypogonadism, myotonia, gastroesophageal reflux disease, and hypothyroidism was scheduled for removal of bilateral ear prosthetic implant magnets under total intravenous anesthesia, as patient was construed to be high–risk for nausea and vomiting.
Pre–operative physical examination and laboratory work–up were unremarkable. Patient was administered midazolam 2 mg intravenously (IV) preoperatively and attached with routine monitors as per ASA guidelines. General anesthesia was induced with inj fentanyl 100 µg, propofol 200 mg (Fresenius Kabi, Sweden) and succinylcholine 60 mg. After smooth induction and intubation, anesthesia was maintained with propofol infusion at 50 µg/min and remifentanil at 0.2 µg/min. After surgical site preparation and time–out, empiric IV cefazolin 2 g was administered. Within a few minutes, patient became severely hypotensive (52/24 mmHg) and bradycardic (46 beats/min). Immediately, glycopyrrolate 0.3 mg, ephedrine 10 mg, and phenylephrine 200 µg were administered, but patient exhibited persistent hypotension and bradycardia. Epinephrine 0.3 mg IV bolus was administered, but to little effect. At this time, surgical procedure was aborted, and anesthetic infusions were discontinued. Patient was placed on an infusion of epinephrine at 3 µg/min and was titrated to maintain a mean arterial blood pressure (MAP) of 60 mmHg. Patient continued to experience severe refractory hypotension to epinephrine infusion and developed bigeminy, ST depression in lateral leads, and prolongation of PR interval. A radial arterial line and an additional intravenous line were secured. Anaphylaxis to medications administered, including cefazolin, was one possibility that came to our minds, so the patient was administered diphenhydramine 50 mg IV, dexamethasone 12 mg and one liter of normal saline. Epinephrine infusion was titrated up to 10 µg/min to maintain MAP of 60 mmHg. A 12–lead electrocardiogram (ECG) showed sinus tachycardia (90 beats/min) and ST depression in lateral leads. Of note, a rash was noticed near IV site along patient’s arm.
After discontinuation of propofol and remifentanil infusions and starting epinephrine infusion, patient’s condition gradually stabilized within 20 min. He was then successfully extubated. While breathing spontaneously on supplemental oxygen, he was transported from the outpatient surgery center to the main hospital post anesthesia care unit (PACU). Perioperative laboratory work–up yielded persistent, severe intractable lactic acidosis (>10 ng/mL; normal results range from 4.5 to 19.8 mg/dL), initially elevated troponin levels, normal tryptase levels, and a glucose spike that gradually decreased (Table 1).
 .
.All subsequent ECG traces were within normal range with complete resolution of ST changes. Cardiology was consulted, and patient was transferred to cardiac care unit for close hemodynamic monitoring and further management. Patient was weaned off of epinephrine infusion over the next 24 hours. The lactic acidosis normalized over the next 48 hours. Patient had normal echocardiogram and negative Lexi scan nuclear stress test. Patient's allergy workup revealed an uneventful cefazolin challenge. Patient declined skin testing to propofol, succinylcholine, and neuromuscular blocking agents (NMBAs). During genetic evaluation, patient reported his mother passed away in her 50s after a relatively low–risk surgical procedure under general anesthesia. The patient refused muscle biopsy as part of MD workup. Patient returned three weeks later for the original surgery. A multidisciplinary team of physicians concluded that the patient suffered atypical propofol infusion syndrome in the face of undiagnosed MD and prescribed an anesthetic plan consisting of ketamine, dexmedetomidine and fentanyl. The patient tolerated that anesthetic and procedure very well, with no adverse events, and was discharged home the same day.
Discussion
MD appears to be the most plausible cause of this perioperative episode of hypotension, bradycardia, and intractable lactic acidosis after propofol administration. The patient’s medical and family history, along with negative allergy workup for the antibiotic used, leave little doubt that the propofol precipitated this clinical presentation. Patient’s history of muscle pain, fibromyalgia, diabetes, congenital deafness, and optic neuritis are consistent with the typical presentation of mitochondrial myopathy.2,3, In particular, because MD is maternally inherited, his mother’s unexpected death from a low–risk surgery under anesthesia raises suspicion of a mitochondrial–linked condition. After the patient was referred for genetic workup and counseling, the final diagnosis suggested was MD. However, patient refused a confirmatory muscle biopsy in order to avoid a similar reaction. Propofol is a mitochondrion–toxin thought to cause acute refractory bradycardia and metabolic acidosis in patients with mitochondrial myopathies.7, 8 Following propofol administration, the patient experienced severe type–B lactic acidosis, hypotension and bradycardia. Finally, patient returned for surgery and had an uneventful postoperative course under a different anesthetic regimen consisting of ketamine, fentanyl and dexmedetomidine, but avoiding propofol.
MD most commonly affects tissues highly dependent on oxidative metabolism, such as the nervous and muscular system.9 Pre-diagnosing and treating MD is a major challenge due to a highly variable clinical spectrum combined with genetic complexity associated with over 120 different mitochondrial DNA deletion types.4,9 Multiple genetic tests, muscle biopsies or brain scans may be required to confirm MD, but many subtypes can still go undiagnosed.4,10 However, surgery and anesthesia expose patients to triggers that can unmask underlying MDs.7 Because general anesthetics have their foremost clinical effect on highly oxygen dependent tissues, patients with MD may have an abnormal sensitivity to intravenous or volatile anesthetics. This may be particularly true with increasing age since bioenergetics appear to progressively depress from adulthood through senescence.9,11 Furthermore, intravenous anesthetic drugs depress properties such as oxygen consumption, energy production, and carbohydrate metabolism in the nervous system of MD patients. Local anesthetics also depress bioenergetics capacity and disrupt oxidative phosphorylation similarly to the mechanism in other intravenous agents.10
Propofol is mitochondrion–toxic, and therefore not safe in high dosages over prolonged periods of time in MD patients.7,12 Propofol markedly decreases oxygen consumption and ATP production in brain synaptosomes, reduces electron flow in cardiac mitochondria, and uncouples electron transport from ATP production.10 A rare complication of propofol is PRIS, which is characterized by acute refractory bradycardia, metabolic acidosis, rhabdomyolysis, myocardial failure, and even death.8,13. One predisposing factor for PRIS is subclinical MD. The physiopathology of PRIS remains unclear, but in animal studies, propofol disrupted electron flow along the respiratory chain and decreased multiple complexes.13 While PRIS occurs more often under long–term propofol infusion (> 48 h) at doses above 4 mg/kg/h, PRIS can be observed with lower doses and shorter duration of sedation.8 Therefore, propofol administration may be problematic in patients with MD.12 Lactic acid is derived from glucose metabolism via the glycolytic pathway and is primarily utilized in the liver and cleared by the kidney. Plasma lactate concentration exceeding 4 mmol/L defines lactic acidosis, categorized into two types.14 Type–A lactic acidosis is caused by impaired tissue oxygenation and hypoperfusion due to shock (septic, cardiogenic, hypovolemic, obstructive), regional ischemia, or seizures. Type–B lactic acidosis is rarer and unrelated to tissue hypoperfusion. Instead, it is due to alternative metabolic pathways for pyruvate, including liver disease, medications, diabetic ketoacidosis, trauma, and MD. This patient’s presentation illustrates type–B lactic acidosis due to MD.
To our knowledge, this is the first case of suspected atypical PRIS due to underlying MD unmasked by a low propofol infusion rate over a very short period of time (around 10 to 15 min). In MD patients, the safest anesthetic regimen remains unknown, but recommendations include avoiding anesthetics such as propofol and succinylcholine,4,5,6 minimally dosing volatiles or intravenous anesthetics, utilizing neuraxial or regional techniques, performing workup of MD (e.g. genetic tests, muscle biopsies), and thorough reviewing of previous anesthetics with patient and family. Alternatives may be considered, such as dexmedetomidine, a selective α2–adrenergic agonist with beneficial effects on mitochondrial membranes in ischemic rats and MD patients in some case reports.15
Conducting clinical trials and establishing evidence–based guidelines for anesthetic management of patients with MD is challenging due to difficulties in diagnosing this rare disease, rendering case reports as the most likely source of information. Much work still needs to be done to determine the safest and most efficacious guidelines for perioperative management of MD. This case report represents an important contribution to the growing body of evidence of anesthetic administration uncovering MDs and consequently formulating safe anesthetics for these patients.
Authors’ contributions
DWK, AH: Literature review, preparation of the manuscript
DV: Provision of anesthetic care, literature review, preparation of the manuscript
GR: Provision of anesthetic care, literature review, preparation of the manuscript, and guidance to primary authors
Conflict of Interest
None declared by the authors. No financial disclosure declared by any of the authors.
References
- Bates MG, Bourke JP, Giordano C, d'Amati G, Turnbull DM, Taylor RW. Cardiac involvement in mitochondrial DNA disease: clinical spectrum, diagnosis, and management. Eur. Heart J. 2012;33(24):3023–3033. [PubMed] DOI:1093/eurheartj/ehs275.
- Srijaya K, Reddy MD, Levy R. Mitochondrial Disease. In: Anesthesia and Uncommon Disease. 6th Philadelphia, Pa: Saunders Elsevier; 2012.433–443
- Miyamoto Yuri, Miyashita T, Takaki S, Goto T. Perioperative Considerations in Adult Mitochondrial Disease: A Case Series and a Review of 111 Cases. Mitochondrion. 2016;26:26–32. [PubMed] DOI:1016/j.mito.2015.11.004
- Maslow A, Lisbon A. Anesthetic Considerations in Patients with Mitochondrial Dysfunction. Anesthesia & Analgesia. 1993;76(4):785–793. [PubMed] DOI:1111/pan.12158
- Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29(9):1417–25. [PubMed] DOI: 1007/s00134–003–1905–x.
- Weinberg G, Baughman V. Carnitine deficiency, mitochondrial metabolism and abnormal response to anesthetics. Anesthesiology. 2006;104:1343. [PubMed] DOI: 1097/00000542–200606000–00036.
- Finsterer J, Frank M. Propofol is Mitochondrian–Toxic and May Unmask a Mitochondrial Disorder. J Child Neuro. 2016;31(13):1489–1494. [PubMed] DOI: 1177/0883073816661458.
- Fodale V, La monaca E. Propofol infusion syndrome: an overview of a perplexing disease. Drug Saf. 2008;31(4): 293–303. [PubMed] DOI: 2165/00002018–200831040–00003.
- Morgan PG, Hoppel CL, Sedensky MM. Mitochondrial Defects and Anesthetic Sensitivity. Anesthesiology. 2002;96(5):1268–1270. [PubMed] DOI: 1097/00000542–200205000–00036
- Khan N, Govindaraj P, Meena A, & Thangaraj K. Mitochondrial disorders: Challenges in diagnosis & treatment. Indian J Med Res. 2015;141(1):13–26. [PubMed] DOI: 4103/0971–5916.154489.
- Muravchick S, Levy RJ. Clinical Implications of Mitochondrial Dysfunction. Anesthesiology. 2006;105(4):819–837. [PubMed] DOI: 1097/00000542–200610000–00029.
- Schieren M, Defosse J, Bohmer A, Wappler F, Gerbershagen MU. Anaesthetic management of patients with myopathies. Eur J Anaesthesiol. 2017;34(10):641–649. [PubMed] DOI: 1097/EJA.0000000000000672.
- Niezgoda J & Morgan, P. Anesthetic Considerations in Patients with Mitochondrial Defects. Paediatr Anaesth. 2013;23(9):785–793. [PubMed] DOI:1111/pan.12158
- Foucher C & Tubben R. Lactic Acidosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019.
- Woodward E & Xiong Z. Use of Methohexital and Dexmedetomidine for Maintenance of Anesthesia in a Patient with Mitochondrial Myopathy: A Case Report. A&A Practice. 2017;8(2):33–35. [PubMed] DOI: 1213/XAA.0000000000000416.