Andrea Trescot, MD
Medical Director, Pain and Headache Center, St. Augustine, FL 32080 (USA)
Correspondence: Andrea Trescot, MD, 4 Oceanside Circle, St. Augustine, FL 32080 (USA); Cell: 904-806-6166; E-mail: DrTrescot@gmail.com
ABSTRACT
Headaches are a common pain complaint, affecting millions of people in the US alone. These pains have been attributed to intracranial processes, such as dilated blood vessels and the trigeminal ganglion, and therefore only amenable to medication therapies. However, many of these headaches, including “migraines”, may be caused by extracranial conditions that can be diagnosed and treated with interventional pain techniques. Using “pattern recognition”, many headache etiologies can be quickly identified, and then treated with ultrasound-guided injections that diagnose and treat the underlying condition. The topics in this article include the diagnosis and ultrasound-guided treatment of supraorbital neuralgia, auriculotemporal neuralgia, and greater occipital neuralgia, and their role in headache management.
Key words: Headache; Migraine; Supraorbital neuralgia; Auriculotemporal neuralgia; Occipital neuralgia; Ultrasound-guided injections
Citation: Trescot A. Ultrasound for evaluation and treatment of headaches. Anaesth Pain & Intensive Care 2017;21(2):241-253
Received: 15 Aug 2015, Reviewed: 2 Jun 2016, Accepted: 28 Jun 2016
INTRODUCTION
Headaches are one of the most prevalent neurologic disorders. They affect 28 million people in the US1 and account for millions of dollars in medical costs, lost labor costs, and associated burdens on our society. As with many medical events, headaches are complex, and our understanding of them is an evolving science. Although often seen as the primary pathology, headaches are fundamentally a symptom and have numerous proven origins and causes. There are many types of headaches, and no two headaches are the same. Despite the individual variations, one can see distinctive patterns of pain that can be easily recognized over and over again. Using pattern recognition, many headaches can be quickly diagnosed, treated, and, with proper care, put to an end entirely. Here, I propose that headaches, including migraines, are not always an isolated intracranial phenomenon. Rather, they can be an interaction between the intracranial components of the brain and the extracranial nerves. In 2003, Pareja et al2 proposed the term “epicrania” for headaches triggered by extracranial causes.
Plastic surgeons at the beginning of this century noted the relief of migraines with both corrugator muscle resection3 and injection of botulinum toxin,4 suggesting a peripheral headache trigger. Headache specialists repeatedly see patients with severe disabling migrainous headaches after a head or neck injury, and these headaches respond only modestly to migraine-specific pain medications. These headaches may have an extracranial origin, and the pain is likely a result of a peripheral nerve irritation and entrapment in the head or neck that was sustained during the injury. Moreover, this peripheral nerve irritation may have secondarily activated the migraine centers in the brain to bring about the associated migrainous symptoms. The treatment, therefore, would be to primarily inhibit the nerve irritation utilizing interventional pain techniques and thus turn off the pain origin, which subsequently turns off the activated migraine centers.
Although these nerves have been evaluated and injected in the past using landmarks and occasionally fluoroscopic images, ultrasound may offer some unique advantages, especially since most of these nerves travel with arteries, perhaps contributing to the complaints of “throbbing” pain.
EXTRA-CRANIAL CAUSES OF “MIGRAINES”, HEADACHES & FACE PAIN
Collectively, headache patterns have provided practitioners and researchers with basic guidelines to further understand and treat this often-debilitating condition. One of the commonly followed classification schemes for headaches is the International Classification of Headache Disorders (ICHD).5 This classification has defined many types of head pain from the viewpoint that headaches are either primary or secondary. The ICHD focuses on the categorization and description of syndromes of headache patterns. While thorough and extremely useful, this lexicon only generally describes certain peripheral nerve contributions to headaches as “Other Terminal Branch Neuralgias.” The ICHD does little to delineate the specific pain patterns of most of the individual extracranial nerve pathologies in the head and neck. The study of the origins of pain, as a function of extracranial peripheral nerve entrapments and their dysfunction, reveals a great deal of overlap between many of the ICHD-defined headaches and the nerve entrapments potentially causing these pain patterns.6 The study of headaches, as a subspecialty in the field of neurology, incorporates evidence from closely associated disciplines such as pain management, which become an invaluable tool in the growing repertoire available to headache practitioners.
SUPRAORBITAL NERVE
Supraorbital neuralgia (SN), first described by Beyer in 19497 is a cause of extracranial headaches, with a reported incidence of 4%.8 SN is defined by the International Headache Society (IHS)9 as a localized headache in the forehead region with the following criteria: paroxysmal or constant pain in the region supplied by the supraorbital nerve.2 SN has multiple etiologies and can sometimes be confused with migraine-type headaches, cluster headaches, or sinusitis. It presents as supraorbital, retro-orbital, and/or forehead pain, unilateral or bilateral, sharp and/or throbbing (Figure1). It may be spontaneous or the result of palpation of the supraorbital area. Occasional patients may complain of blurred vision, nausea, and photophobia, thereby confusing the diagnosis.10 Triptans can help but usually only temporarily, presumably by vasoconstricting the blood vessels travelling with the nerve (Figure 2), thereby decreasing the entrapment. Symptoms include continuous or intermittent unilateral pain above the eye that occasionally radiates distally along the SON to the vertex of the skull.
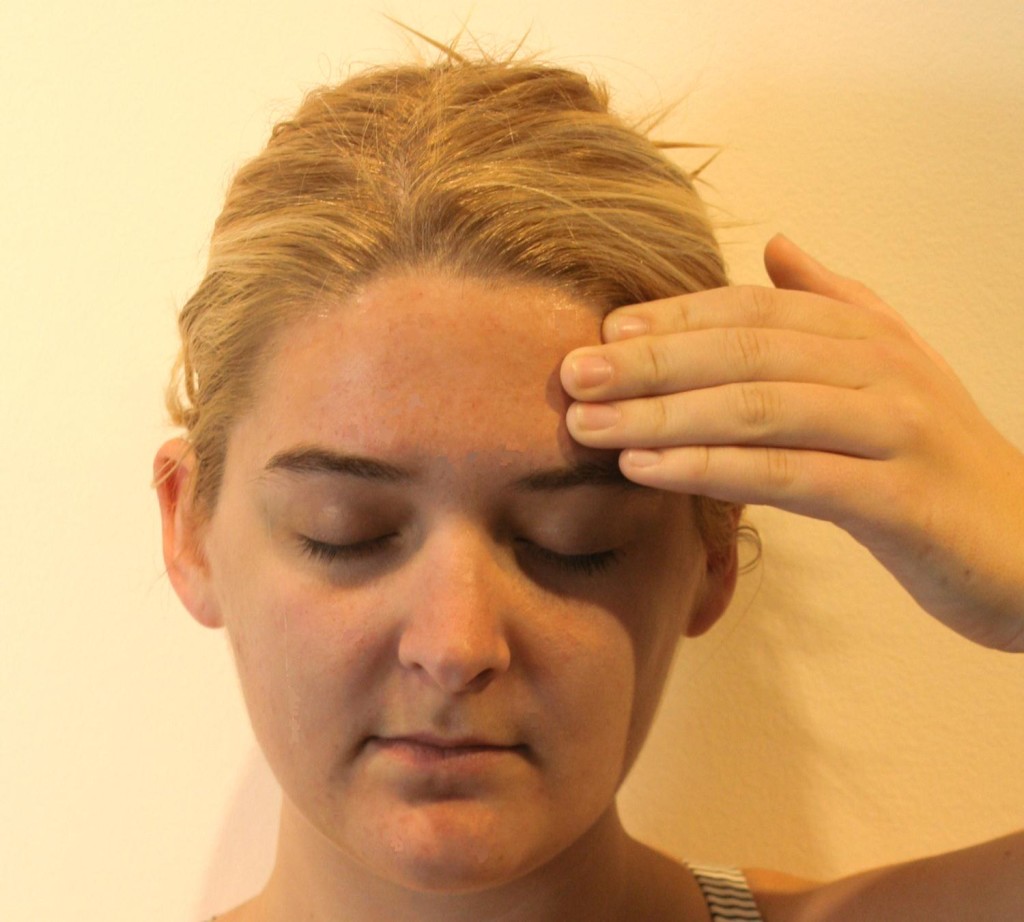
Figure 1: Patient pain complaint from supraorbital nerve entrapment. (Image: Andrea Trescot, MD)
The SON can also serve as a trigeminal “trigger zone” for tic douloureux. The symptoms may worsen with time, especially if they are associated with progressive scarring around the nerve. Headache symptoms are often exacerbated with excessive squinting or frowning (when the orbicularis oculi entraps the nerve), direct pressure (such as with swimming goggles), the head held lower than the heart (because of increased blood flow), or fluid retention associated with pre-menses and excessive salt consumption (which causes swelling of the nerve in its canal). All these factors can lead to further compression of the nerve.
Like hemicrania continua and cluster headaches, the headache from supraorbital nerve entrapment can have associated ipsilateral conjunctival injection, lacrimation, nasal congestion, rhinorrhea, and frequent ipsilateral conjunctival injection, lacrimation, nasal congestion, rhinorrhea, and frequent “attacks.” In fact, hemicrania continua has been successfully treated with supraorbital and supratrochlear nerve injections.11 And as with migraines, there may be associated photophobia, phonophobia, nausea, and emesis. The pain is almost always unilateral and can be severe enough to lead to suicidal thoughts.12
Anatomy
The SON is one of five peripheral branches of the ophthalmic division of the trigeminal nerve (cranial nerve V). The trigeminal ganglion (TG) gives rise to three divisions, ophthalmic (V1), maxillary (V2), and mandibular (V3). While the V2 and V3 divisions branch downward, the V1 division branches superior and medially from the TG until it enters the orbit posteriorly via the superior orbital fissure. The nerve then divides into 3, continuing on as the lacrimal nerve, the nasociliary nerve, and the frontal nerve. The frontal nerve then splits into the supraorbital nerve (SON), which exits the orbit with the supraorbital artery via the supraorbital notch (Figure 2), and the supratrochlear nerve (STN), which exits with the supratrochlear artery via the supratrochlear groove or notch (Figure 2). and runs medially in a small groove at the junction of the ocular ridge and the nares. The SON then branches into a medial and lateral branch over the forehead (Figure 3).
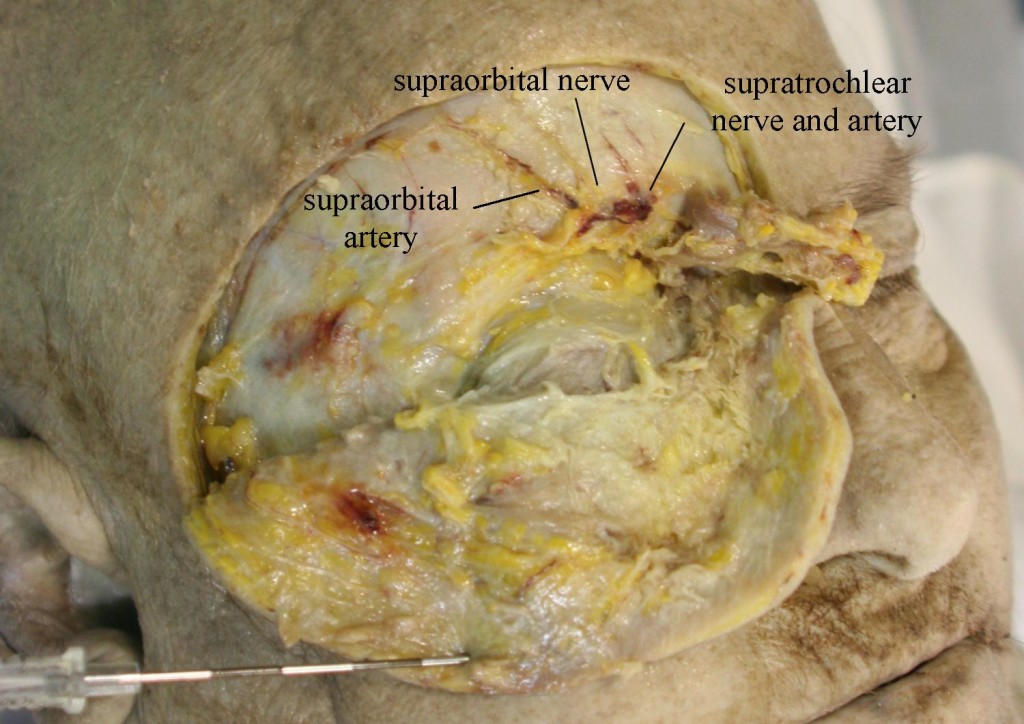
Figure 2: Supraorbital nerve and artery dissected
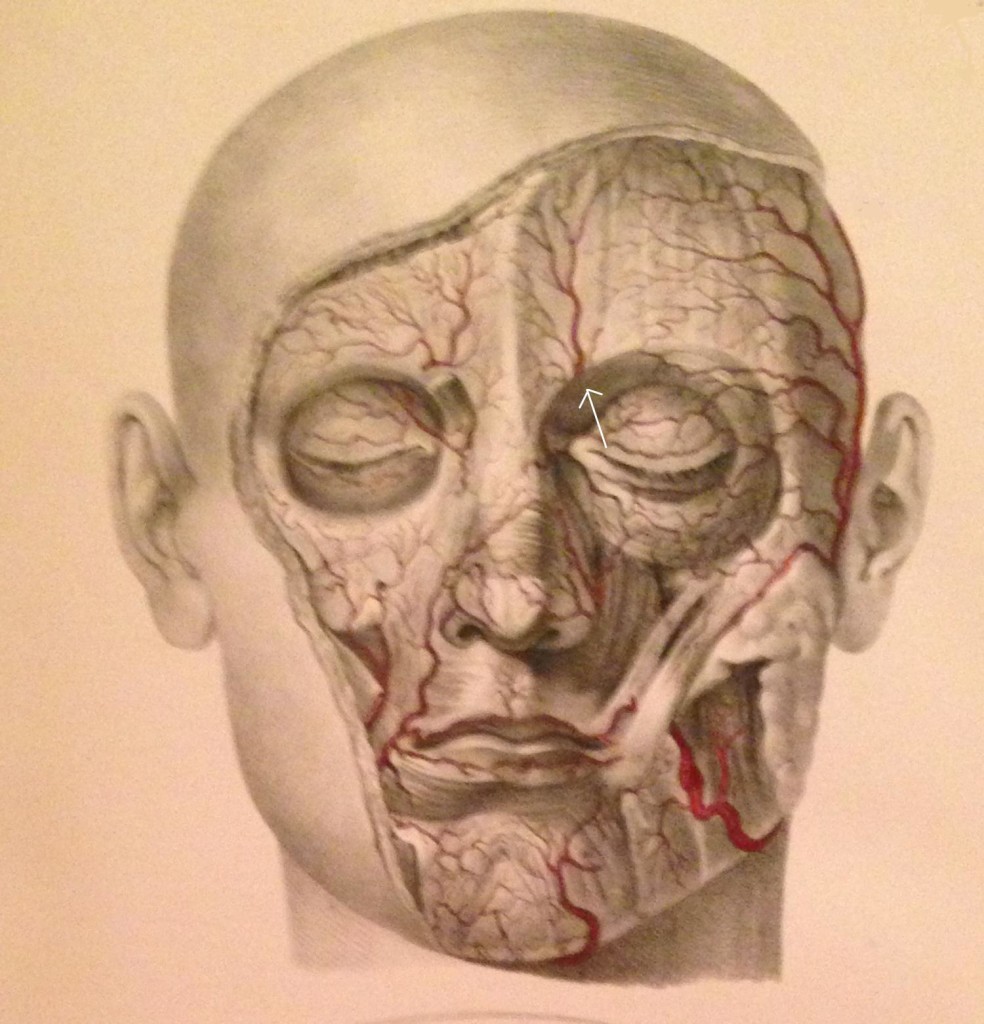
Figure 3: Facial arteries; arrow identifies the supraorbital artery (Image from Quain J, Wilson WJE. The Vessels of the Human Body, 1837)
Separate exits for the medial and lateral SON branches were observed in eight of the 28 nerves (29%) examined by Anderson et al.13 The medial branch continues superiorly up to the vertex of the skull, while the lateral branch moves superior-laterally; both branches supply sensory fibers to the forehead, upper eyelid, and anterior scalp, up to the lambdoidal suture, and both were associated with arteries (often intimately entwined). Janis et al. found that 73% of the specimens they examined had the SON passing through the corrugator muscle.14
The supraorbital notch may be covered by a small ligament (creating a foramen), which can become thickened or calcified (Figure 4).
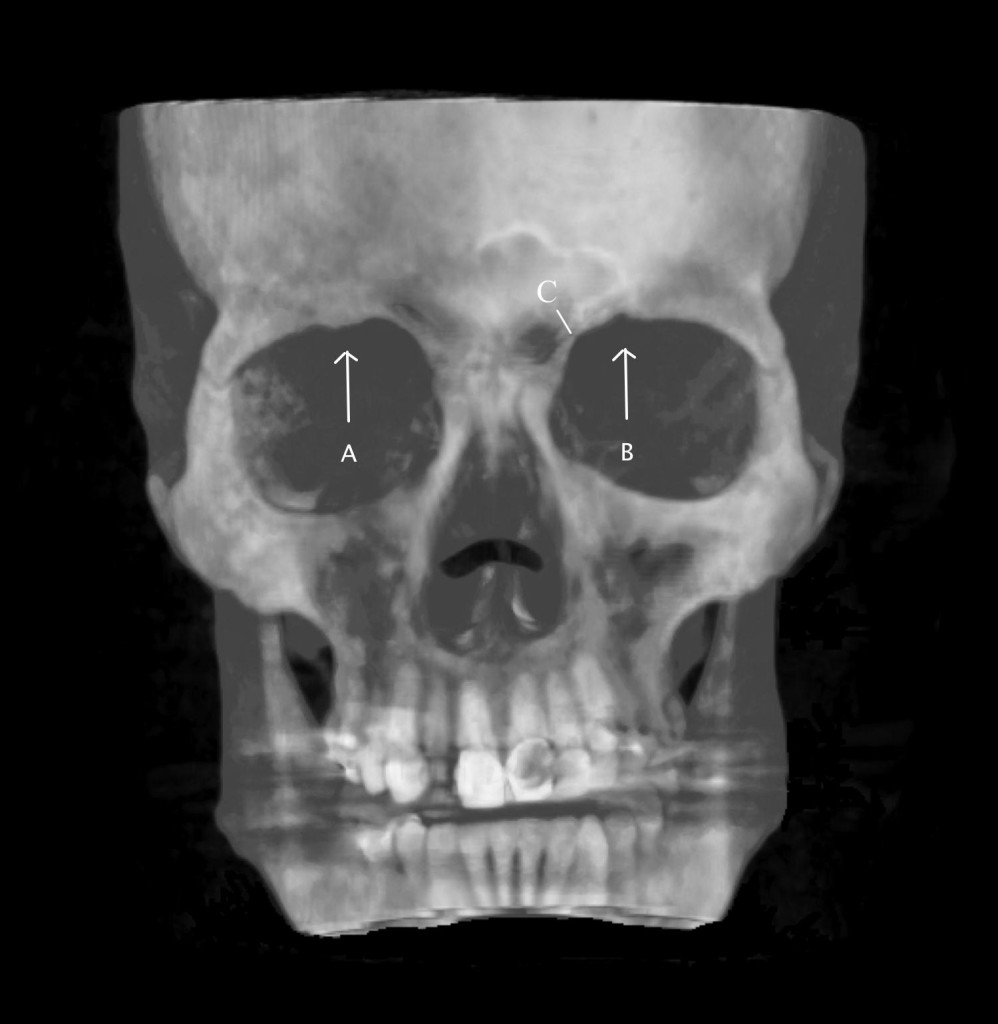
Figure 4: 3D image of the skull. Arrows show supraorbital notch. A = obliterated foramen, B = supraorbital notch. C = supratrochlear groove. (Image: Andrea Trescot, MD)
Fallucco and colleagues called this ligament-covered foramen a “miniature carpal tunnel”.15). In dissections done by Anderson et al.,13 20 of 28 nerves (71%) exited through foramina (with bony or connective tissue bridges) instead of notches. Fallucco et al.15 dissected 50 SONs and found that 43 of the 50 (86%) had fascial bands across the notch. These were further divided into “simple” bands (22 of 43 or 51.2%), “partial bony” (13 of 43 or 30.2%), and ‘septums’, which could be horizontal (4 of 43 or 9.3%) or vertical (4 of 43 or 9.3%). There was a high degree of variability, and the authors noted that the anatomic difference and variable diameters could account for unilateral migraine symptoms.
Entrapment
Entrapment occurs primarily at the supraorbital notch as the nerve leaves the orbit, passing through the supraorbital notch, the orbicularis oculi muscle, the glabellar muscle, and the corrugator muscle, and it can be exacerbated by frowning and squinting (perhaps the reason that some “migraines” respond to use of eyeglasses or botulinum toxin). Tight fitting goggles16 or an anesthesia mask17 can compress the nerve. There can also be trauma to the nerve from surgery in the frontal region,17 This headache will often worsen with menses or increased salt intake, perhaps by causing swelling of the nerve in its canal. In addition, there can be entrapment by scarring17 as well as by thickening or calcification of the supraorbital ligament.
Physical Examination
A thorough history should first be obtained to identify any previous trauma or inciting events that could lead to SN. This is then followed by a visual inspection and physical examination of the forehead, eyebrow, nasal region, and supraorbital foramen. There will be tenderness to palpation over the supraorbital foramen with possible radiation of symptoms along the nerve distribution of the affected side. Support the head with the non-examining hand, then palpate over the orbital rim, feeling for the supraorbital notch (Figure 5). Parasthesias should replicate the pain complaints, and, with careful palpation, the examiner should be able to identify the slim, string-like vertical SON structure. Move the thumb medially to feel the supratrochlear groove.
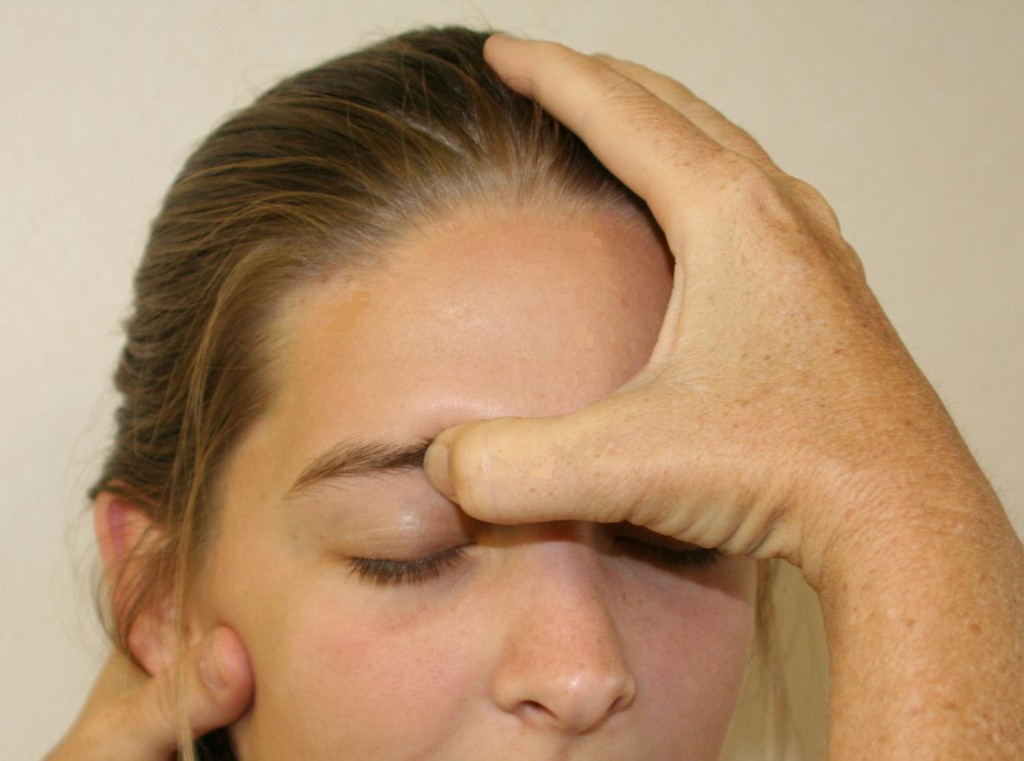
Figure 5: Physical examination of the supraorbital nerve. (Image: Andrea Trescot, MD)
In 2005, Sjaastad and colleagues reported on the supraorbital examination of 1,828 of the inhabitants of Vågå, Norway.12 Only 24 persons (1.3%) did not have a palpable SON; 98% of the individuals had a palpable SON, and 5.4% of the inhabitants (106 of the 1,828 people examined) had increased tenderness over the supraorbital notch. Twelve of these had a clinical presentation of SN; 10 of the 12 had a history of forehead trauma.
Ultrasound Examination
Using ultrasound (US) to block the supraorbital nerve can be beneficial over the landmark-guided or fluoroscopic approaches. The linear probe is placed horizontally across the supraorbital notch (Figure 6). With direct visualization of the supraorbital foramen, proper needle placement can be achieved as well as avoiding inadvertent needle placement into the foramen. In a recent cadaveric study, Spinner and colleagues18 demonstrated that ultrasound-guided blocks of the supraorbital and infraorbital nerves had greater accuracy than conventional landmark-based techniques. The results of the study showed that the US accuracy rate was 100% (18 of 18) for the in-plane approach and 94% (17 of 18) for the out-of-plane approach. Thirty-five injections were considered accurate (97%) with overflow, and one injection was inaccurate. The study concluded that ultrasound-guided injections had a higher degree of accuracy versus the standard techniques used today. The US technique would be performed the same as the conventional landmark-guided technique with improved visualization of the target.
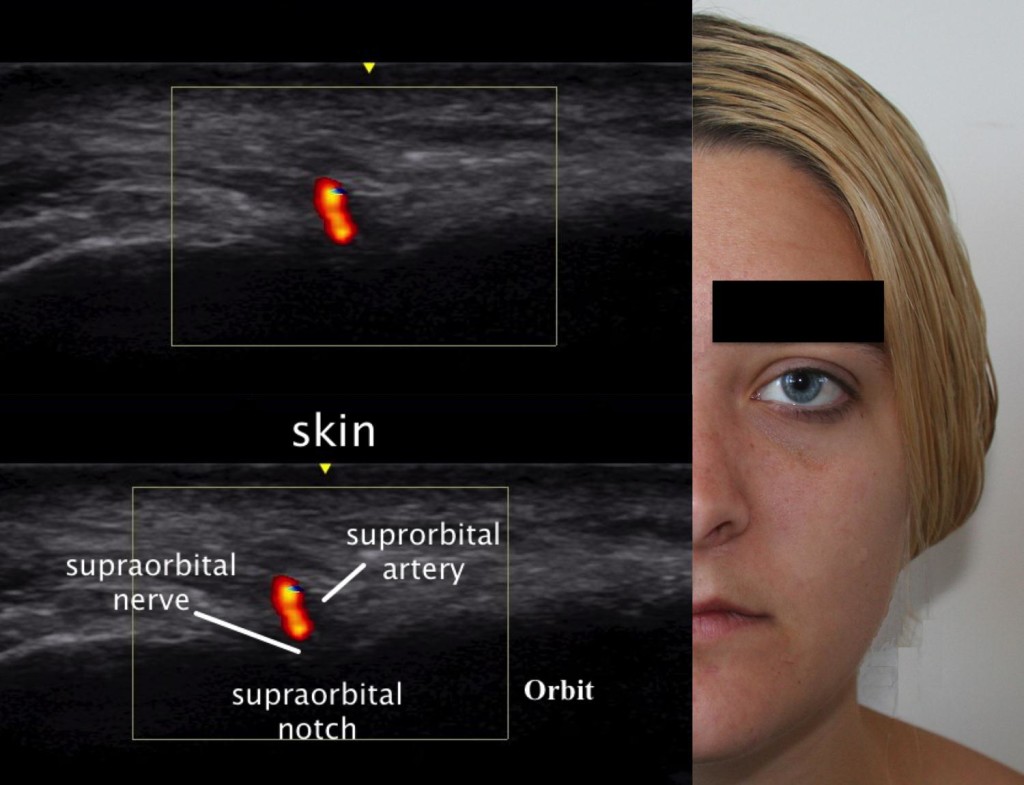
Figure 6: Ultrasound image of the supraorbital nerve. (Ultrasound Image: David Spinner, MD; composite created by Andrea Trescot, MD)
AURICULOTEMPORAL NEURALGIA
Temple headaches may be due to entrapment of the auriculotemporal nerve (ATN), a third-division branch of the trigeminal nerve. Other areas of pain when this nerve is entrapped may include pain in the auricular area and the temporomandibular joint. This is a common headache site (just visualize all the headache patients rubbing or pressing their temples for relief) shared with migraine headaches, and it may often be misdiagnosed as an intracranial migraine. There are two distinct clinical presentations of ATN nerve dysfunction, and a variety of names, including auriculotemporal neuralgia, auriculotemporal syndrome, Frey syndrome, Baillarger syndrome, Dupuy syndrome, salivosudoriparous syndrome, and gustatory sweating syndrome.
Clinical Presentation
Entrapment of the ATN is probably the most common of the trigeminal headaches. Calandre et al.19 evaluated specific areas in the scalp that could be manually palpated to elicit a headache; 42.6% of these “trigger points” were found in the temple region and 33.4% in the suboccipital region. However, ATN entrapment is rarely diagnosed and represented only 0.4% of the referrals to a tertiary headache outpatient clinic.20
There are two main presentations of irritation of this nerve: Auriculotemporal Neuralgia (ATn) and the rarer Auriculotemporal Syndrome (ATS), both of which can have overlapping presentations, as well as multiple etiologies.
ATn typically presents as paroxysmal attacks of pain in the preauricular and temple regions as well as the retro-orbital region and the teeth.21 The patient with ATn headaches due to ATN entrapment will often awaken at 3-4am with a headache in the auricular area that radiates into the temple (Figure 7) and the retro-orbital as well as the occipital region.

Figure 7: Patient’s description of pain from auriculotemporal neuralgia. (Image: Andrea Trescot, MD)
The headache may be unilateral22 or bilateral, and may have associated ear, parotid, and jaw pain or numbness.20 The headache is often throbbing in nature, possibly due to its proximity to the temporal artery. There is often increased pain with talking, chewing, and menses.23 The intensity of the pain is moderate to severe, and the quality is usually constant, though at times there may be paroxysms of sharp, jabbing, lancinating pain or a throbbing, pounding pain. The pain may be triggered by trauma to the jaw or temple, as well as by surgery, exacerbated by palpation over the preauricular area or by chewing, and can be associated with severe nausea and vomiting. The pain characteristics and accompanying nausea and vomiting meet the International Headache Society criteria9 for a “migraine”, though there is no specific listing for ATn. The early morning headache appears to be related to bruxism or nocturnal jaw clenching. Because there is a physical connection with the facial nerve (see Anatomy section), there can also be pain in the muscles of facial expression with ATN entrapment.
In 2005, Speciali and Gonçalves 20 evaluated six patients with ATn proven by diagnostic nerve blocks (see Injection techniques section). All were females with unilateral pain, suffering with symptoms from 1 month to 20 years, and all of the patients had pain with palpation of the preauricular area just above the tragus. Pain was primarily around the ear, radiating to the head of the mandible and temple in all of the patients. One patient had knife-like pain triggered by gustatory stimuli (see Frey’s syndrome below). In 4 patients, the pain radiated to the occipital region, but there was no relief from occipital nerve blocks (see below). There were complaints of facial tingling or parasthesias in 4 patients, but, notably, temporomandibular joint (TMJ) pathology was absent in all of the patients. All of the patients had complete or near-complete relief with ATN injections.
Murayama et al.21 described a patient with left ear pain and ipsilateral maxillary second molar pain. The dental examination was normal, and the tooth and ear pain resolved with a local anesthetic and steroid injection of the ATN, with remission still present 6 months later.
The Auriculotemporal Syndrome, also known as Frey syndrome, was described by Frey in 192324 as a constellation of symptoms including unilateral hyperhidrosis and flushing of the cheek and ear that occurs when eating or drinking anything that stimulates the parotid gland to produce saliva (Figure 8). Pain is uncommon, but there may be sensory changes in the ATN distribution, and there may be a concomitant trigeminal neuralgia. Also known as Baillarger syndrome, Dupuy syndrome, salivosudoriparous syndrome, and gustatory sweating syndrome, these symptoms usually occur 2 to 13 months after surgery, open trauma, or infection of the parotid gland, though it has also be described after dislocation of the mandibular condyle.25 The proposed cause is an improper regeneration of the sympathetic and parasympathetic nerves of the parotid gland, and the syndrome occurs in about 5% of surgical patients, even with careful technique. The severity of symptoms associated with Frey syndrome can range from mild to debilitating.
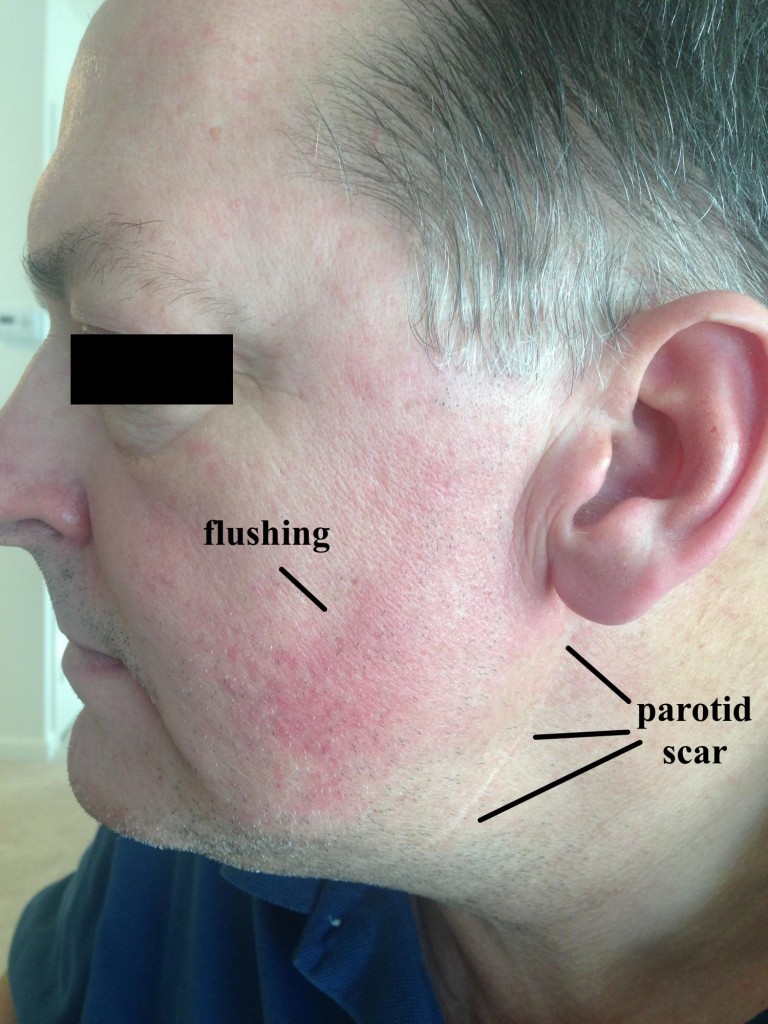
Figure 8: Frey syndrome. Note the well-healed parotid scar and the facial flushing with eating.
Gordon and Fiddian26 reviewed the records of 71 patients who had parotid surgery; 17 patients had “noticeable” Frey syndrome. Fourteen patients had positive Minors’ test (iodine/starch), with the greater auricular nerve involved in 6 cases, the ATN in 4 cases, and both in 2 cases (with 2 inconclusive tests). Choi et al.27 evaluated 59 patients after parotidectomy, using subjective symptoms, Minor’s starch iodine test, and thermography, and found a “good” correlation between the 3 indicators. Pain triggered by taste stimuli can be a significant component of Frey’s syndrome; Scrivani et al.28 described 6 patients with recurrent, episodic shocking facial pain triggered even by the smell of food, which occurred days to weeks after head and neck surgery.
Anatomy
The ATN is a branch of the third division (mandibular) of the trigeminal ganglion. The mandibular nerve exits the cranium through the foramen ovale and then immediately divides into an anterior and posterior division, providing sensation to the TMJ, tragus, external auditory canal, parotid, base of the auricle, and skin of temporal region (Figure 9).
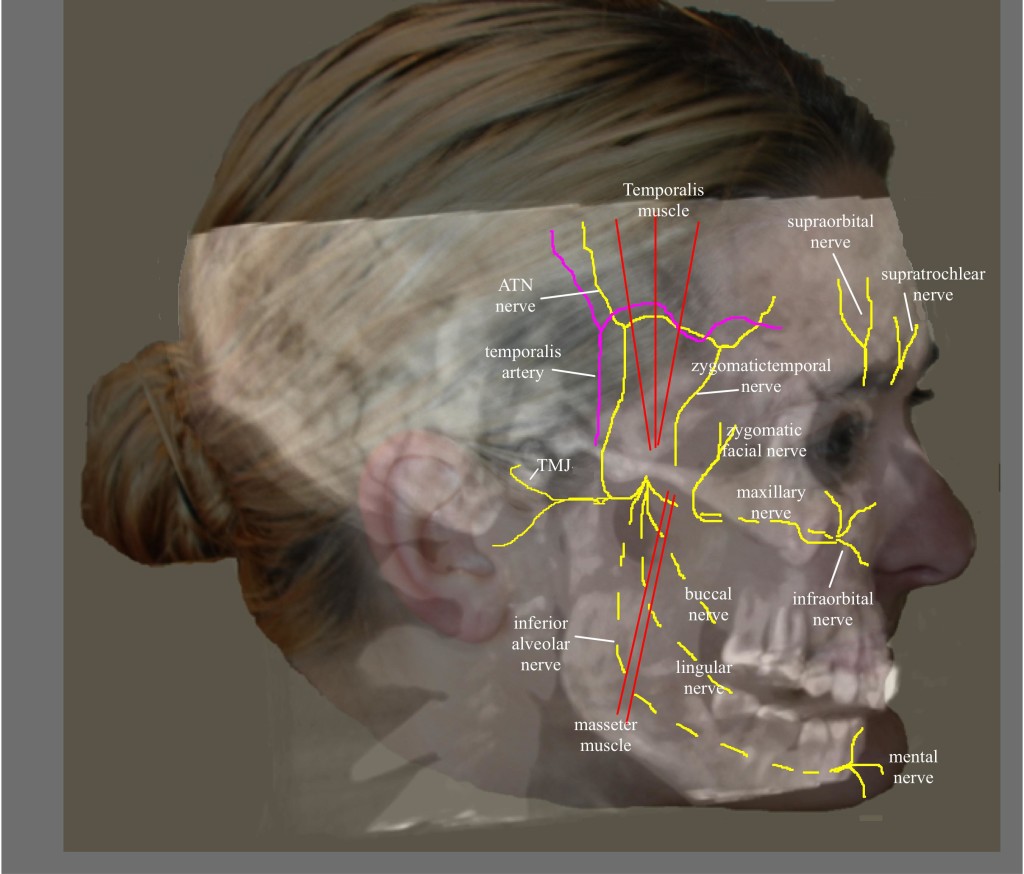
Figure 9: Nerves of the face. (Image: Andrea Trescot, MD)
The anterior division travels between the roof of the infratemporal fossa and the lateral pterygoid muscle (LPM) and is composed of the anterior deep temporal nerve, the posterior deep temporal nerve, and the masseteric nerves. The posterior division consists of the lingual nerve, inferior alveolar nerve, and ATN, descending medial to the LPM.29 Because the ATN has a long and tortuous course, it is at risk for irritation and entrapment. The ATN arises by two roots, which travel on either side of the ascending middle meningeal artery and then join together to form a short trunk. It leaves the foramen ovale, and runs beneath the pterygoideus externus to the medial side of the neck of the mandible. It then turns upward with the superficial temporal artery, between the external ear and condyle of the mandible, under the parotid gland. After it leaves the gland, it ascends over the zygomatic arch, traveling in front of the temporal mandibular joint (providing the primary enervation of the joint as it goes by) to pierce the temporalis muscle. The ATN then divides into five small branches, including the nerve to the external auditory meatus, which innervates the skin of the meatus and the external tympanic membrane; the parotid branch nerves; the articular branches, which innervate the posterior part of the TMJ; and the superficial temporal nerve, which innervates the skin of the temporal region. The superficial temporal nerve communicates with the facial nerve, the zygomaticotemporal nerve (which is a branch of the maxillary nerve) and zygomatic branches of the facial nerve.
The ATN has been associated with migraine headaches. Chim et al.30 dissected the ATN in 20 cadavers and found 3 potential compression points along the path of the nerve. Point 1 and 2 corresponded to fascial bands, with point 1 being 13.1 ± 5.9mm anterior and 5 ± 7mm superior to the superior point of the external auditory meatus and point 2 found at 11.9 ± 6mm anterior and 17.2 ± 10.4mm superior to the most anteriosuperior portion of the external auditory meatus. The 3rd point represented the interaction of the ATN and the superficial temporal artery (Figure 10), found in 80% of the dissection. There were 3 types of interactions noted – the artery crossing over the nerve (62.5%), the nerve crossing over the artery (18.8%), or the artery wrapping helically around the nerve (18.8%).
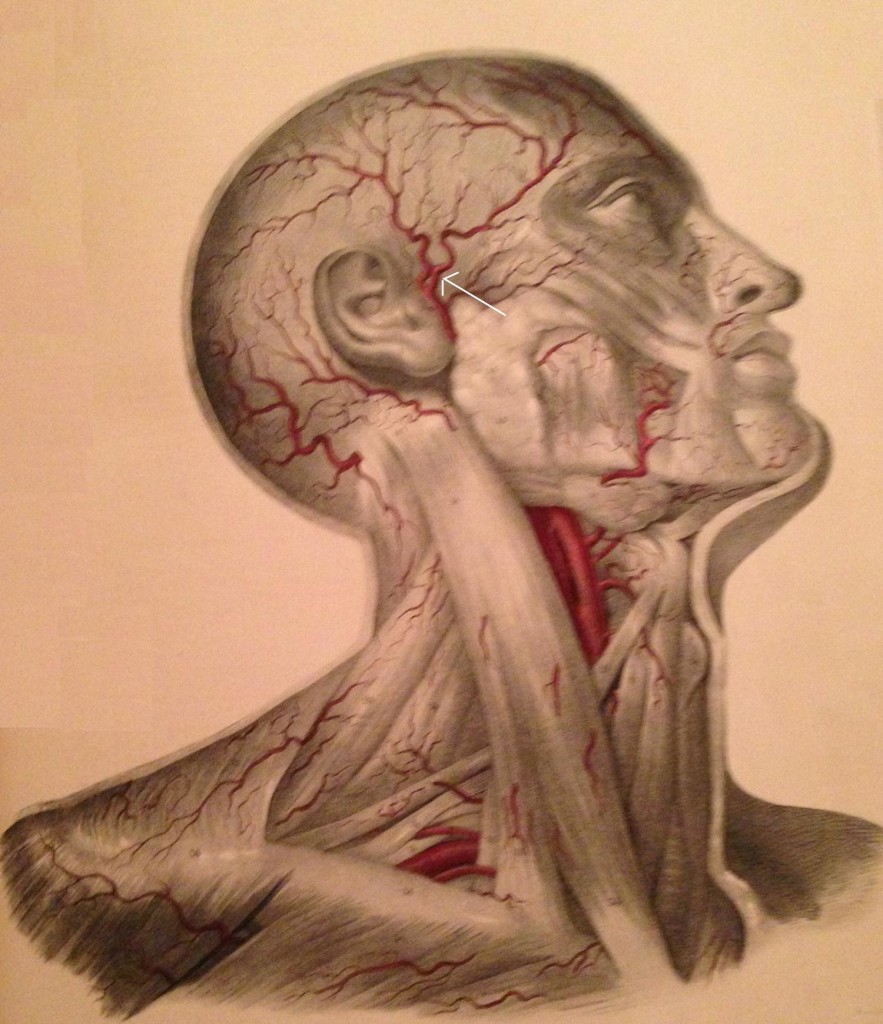
Figure 10: Temple arteries; arrow identifies the temporal artery (Image from Quain J, Wilson WJE. The Vessels of the Human Body, 1837)
Andersen et al.13 dissected 10 heads and found the superficial ATN branch to be located between 8 and 20mm anterior to the root of the ear helix. Only 4 of the cadavers had a single branch anterior to the tragus; the rest had multiple branches. The temporal artery was always found deep to the ATN, and in 3 dissections the ATN connected with the temporal branch of the facial nerve. There was noted to be a connection between the ATN and lesser occipital nerve in 2 cases, and between the ATN and the greater occipital nerve in 4 cases.
Janis et al.1 dissected 50 cadaver temples and identified that the ATN was always found to lateral to the artery in the upper preauricular temple. In another 50 cadavers, Janis et al.31 dissected the periorbital and temporal regions, evaluating the path of the zygomaticotemporal branch of the ATN. In “exactly half” of the specimens, the nerve had no intramuscular path; in the other half of the cadavers, 11 had only a brief course through the muscle, but 14 had a long, tortuous path through the muscle, making entrapment likely.
Entrapment
Entrapment of the posterior division of the mandibular nerve is not uncommon,20 with one study showing entrapment in 6% of 52 cadavers, primarily at the level of the LPM.29 Another study of 10 cadaver dissections showed myositis, local ischemia, and inflammation of the LPM in patients with premortem complaints of pain in an ATN distribution.32 Spasm of the LPM or local changes within the muscle can entrap the nerve, causing numbness of the external ear and TMJ.29 The ATN can also be entrapped between the medial and lateral pterygoid muscles, causing facial numbness, mandibular pain, or headaches.33
The anatomic relationships between the ATN and the muscles of mastication, the TMJ, and the surrounding vascular structures set up the potential for entrapment, so that ATn can play a role in TMJ pathology, headaches, and external ear pain. Secretomotor fibers within the parotid gland may also be entrapped, leading to impairment of salivation (and therefore triggering Frey syndrome).
Janis et al.1 proposed an entrapment of the ATN by the superficial temporal artery, finding a “direct relationship” between the ATN and the superficial temporal artery in 34% of the cadavers studied, with the ATN wrapped helically around the superficial temporal nerve. Because of the ATN and facial nerve connection, entrapment of the nerve at this level can cause pain in the muscles of facial expression.29
In addition, the temporal artery entraps the zygomaticotemporal nerve (a branch of the maxillary nerve that exits through the zygomaticotemporal foramen and innervates a small area of the forehead and temporal region), mimicking an ATN.34
Physical Examination
Since there are several sites of entrapment, the physical examination must be able to separate each area of pathology. Examination should include the TMJ (placing the examining fingers over the joint as the patient opens and closes their mouth), the masseter (rolling the examining thumb horizontally as the patient clenches their jaw) and the zygomatic branch of the facial nerve (placing the examining thumb at the anterior condyle of the mandible, just under the zygoma). The examiner should also palpate of the temporalis muscle (looking for trigger point tenderness) and the superficial temporal artery (to evaluate for temporal arteritis).
The most clinically common site of entrapment for the ATN is in the temporal fossa; Trescot described finding the ATN by placing the index finger at the apex of an isosceles triangle created by resting (for the right-sided exam) the thumb on the tragus and the middle finger on the canthus (corner of the eye) (Figure 11).23
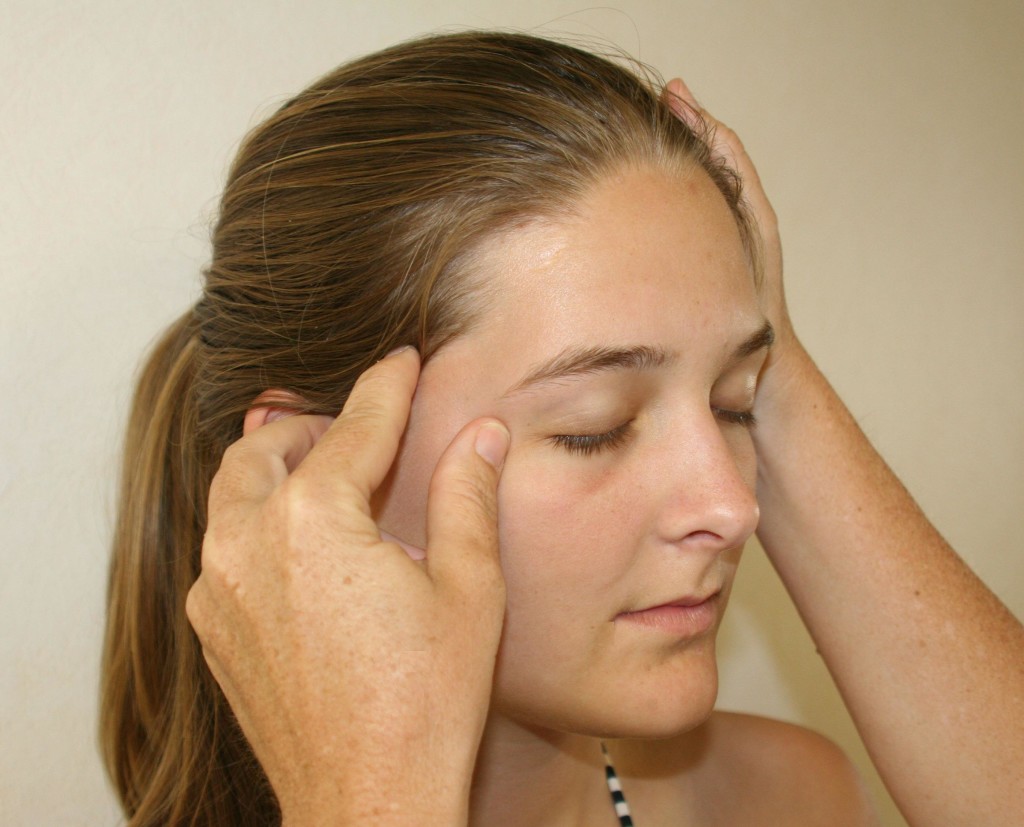
Figure 11: Auriculotemporal nerve examination. (Image: Andrea Trescot, MD)
Ultrasound Guided Injection
In 2007, Shankar and Brethauer35 described an US technique for the ATN injection. The US probe is placed transversely just above the TMJ, and color Doppler used to identify the superficial temporal artery; the nerve appears as a small hyperlucent bundle (Figure 12A). The probe is then rotated to perform a longitudinal scan to track the nerve cephalad (Figure12B). They used a 25g needle from an out-of-plane approach to deliver medication (2cc of 1% lidocaine and 10mg of Depo-Medrol®) around the nerve.
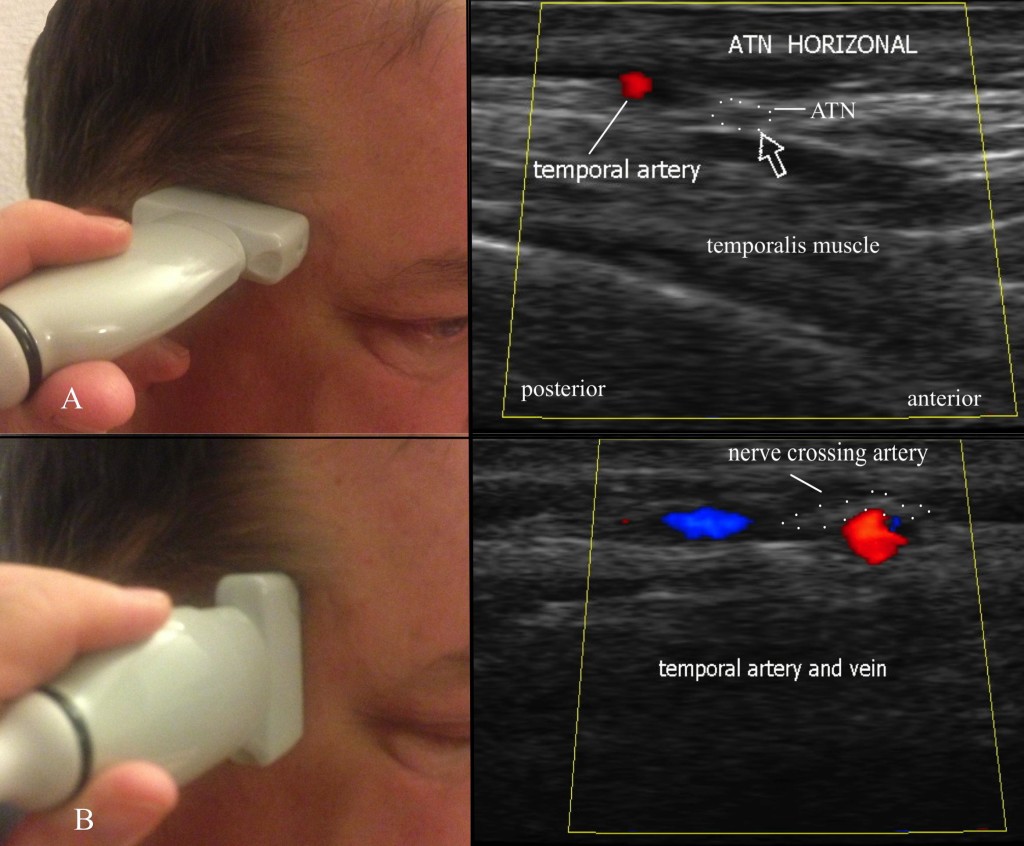
Fig 12: Ultrasound evaluation of the auriculotemporal nerve. A = horizontal evaluation; B = vertical evaluation. (Images: Andrea Trescot, MD)
Because of its tortuous path, its proximity to a significant artery (the temporal artery), and its frequent misdiagnosis, US is an excellent tool for the diagnosis and treatment of the ATN
OCCIPITAL NERVE
The greater occipital nerve (GON) entrapment commonly causes occipital and cervicogenic headaches, and can sometimes cause headaches with characteristics similar to migraine headaches. The anatomic recognition of multiple occipital nerves (the greater occipital, the lesser occipital, and the third occipital nerve), each with different patterns of pain, neurologic origin, and treatment modalities, has been facilitated by the use of precision diagnostic injections. This article will focus specifically on the GON. There are multiple names used for these nerve entrapments, including “migraine”, “tension headache”, “cervicogenic headaches”,36 and “occipital myalgia-neuralgia syndrome”.37 Sometimes the term “suboccipital injections” has been used to describe greater occipital nerve injections, but, for this article, I use “suboccipital” to mean the C1 nerve root or the high volume occipital decompression. Occipital neuralgia is also known as C2 neuralgia or Arnold’s neuralgia.
Clinical Presentation
Beruto et al.38 described occipital neuralgia (ON) in 1821 as a sharp, lightening-bolt pain radiating from the occiput to the vertex. The medical terms occipital neuralgia and cervicogenic headache describe a syndrome of neck and head pain primarily referring to the occiput, as well as the temporal area, forehead, and retro-orbital areas, that may arise some distance away, in the upper cervical spine. Greater occipital neuralgia characteristically presents as paroxysmal shooting, stabbing pain from the suboccipital region to the vertex (Figure 13). The cutaneous innervation of the posterior neck and occiput comes primarily from C1, C2, and C3, which provide overlapping dermatomes (Figure 14). C2 covers the occiput, neck, and submental regions, while the C3 dermatome can span from the clavicle to the mandible to behind and over the ear, pinna, and the angle of the mandible.39 Since the greater occipital nerve is made up of contributions from C1, C2, and C3, there can be a wide range of clinical presentations.

Figure 13. Pattern of greater occipital pain. (Image: Andrea Trescot, MD)
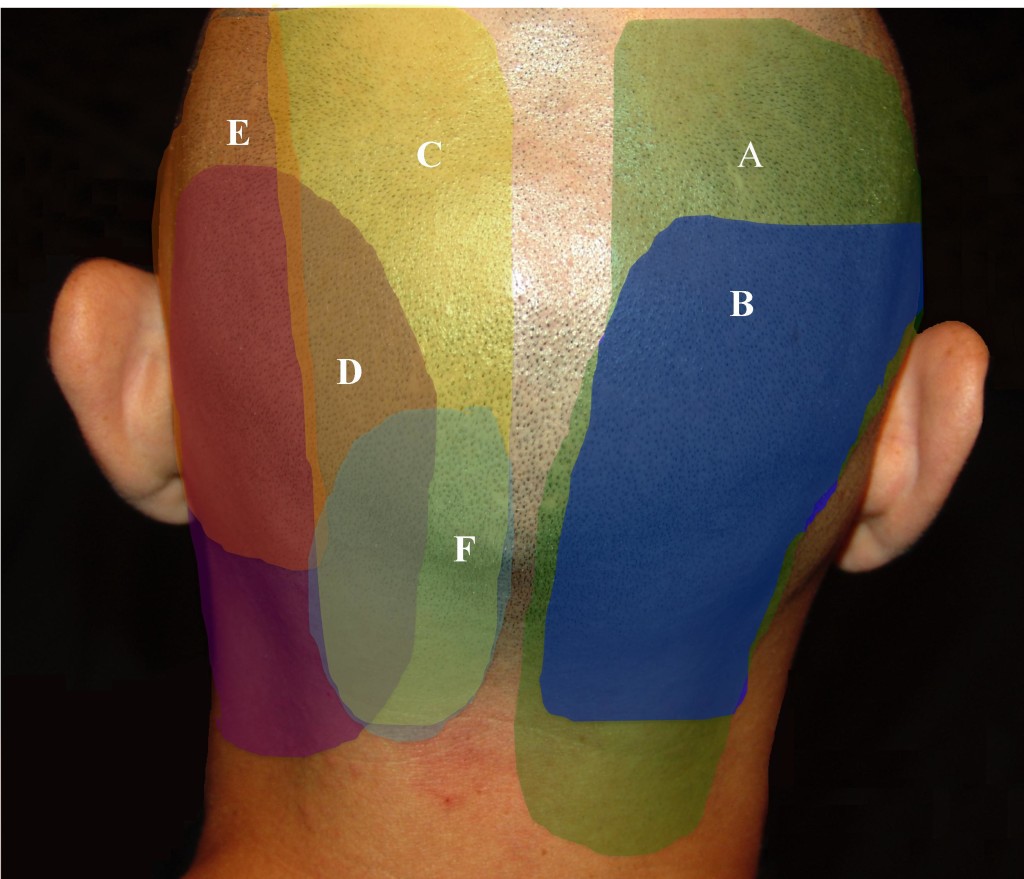
Figure 14. Pattern of posterior cervical and occipital pain. A = C2/3 facet; B = C3/4 facet; C = greater occipital nerve (GON); D = posterior auricular nerve (PAN); E = lesser occipital nerve (LON); F = third occipital nerve (TON). (Image: Andrea Trescot, MD)
As a subset of cervicogenic headaches (CGH), occipital neuralgia can cause pain and paresthesias to the posterior scalp, the periorbital, temporal and mandibular regions, the external ear and mastoid regions, as well as pain in the neck and shoulders. The first three cervical spinal nerve segments (C1-C3) that make up the occipital nerves share a relay-station in the brainstem that continues into the upper cervical spinal cord with the trigeminal cell bodies (the cervico-trigeminal complex). The pain of occipital neuralgia and cervicogenic headaches can therefore be referred to structures innervated by the branches of the trigeminal nerve, namely, the forehead, temples, and eyes.
There are three occipital nerves – the greater, the lesser, and the third occipital nerves. Their patterns of pain are overlapping, and so the reader is encouraged to review information on these nerves as well.
Unilateral ON, perhaps because of the proximity of the occipital artery (see below), can present with throbbing, unilateral headaches associated with photophobia, phonophobia, and nausea; symptoms meet the International Classification of Headache Disorders (ICHD) of a “migraine”.5 There may be a history of occipital pain, often radiating to the face, specifically described as “behind my eye” and “like an ice pick”. Occipital headaches may start as “tension headaches” in the upper cervical region, but then center at the base of the skull and produce throbbing, unilateral or bilateral pain, accompanied by nausea, photophobia, and phonophobia. Occipital neuralgia has a close relationship with cluster headaches, with several authors40-42 describing the use of occipital blocks to treat cluster headaches. Hemicrania continuum43 and “transformed migraines”44 have also responded to occipital nerve blocks.
Anatomy
The largest of the three occipital nerves, the GON arises from the posterior ramus of C2 that runs inferiorly between the arch of C1 (atlas) and the lamina of C2 (axis), lateral to the lateral atlantoaxial (AA) joint, deep to the inferior oblique muscle. The GON then curves up over the inferior oblique, between the inferior oblique and the semispinalis capitis muscles (Figure 15).
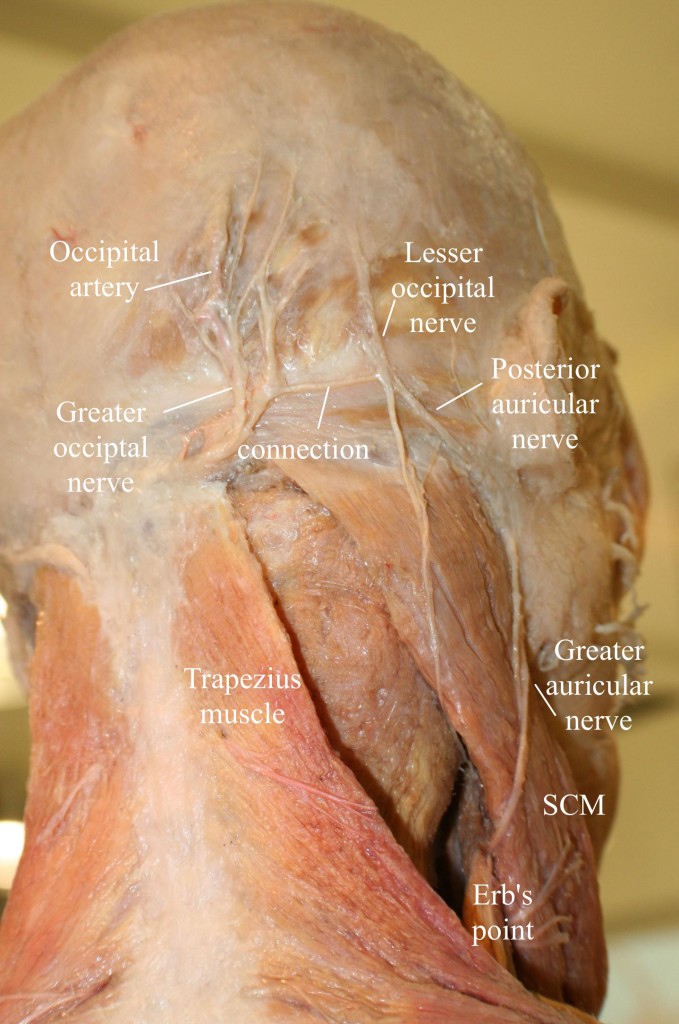
Figure 15: Anatomy of the occipital region, modified from an image from Bodies, The Exhibition, with permission. Note the connection of the greater and lesser occipital nerves. (Image: Andrea Trescot, MD)
A branch from C3 may join at this point, as the nerve ascends up the neck and over the dorsal surface of the rectus capitis to pierce the semispinalis capitis muscle, deep to the trapezius muscle. The GON then exits the neck through a muscular sling formed by the aponeurosis of the sternocleidomastoid muscle (SCM) and trapezius muscle at their attachment on the occipital bone (the conjoined tendon), where it is joined laterally by the occipital artery. At this point, the greater occipital nerve is immediately medial to the occipital artery and lateral to the inion or occipital prominence, lying in a palpable groove. This proximity to the artery may account for the throbbing sensation that often accompanies GON entrapment.
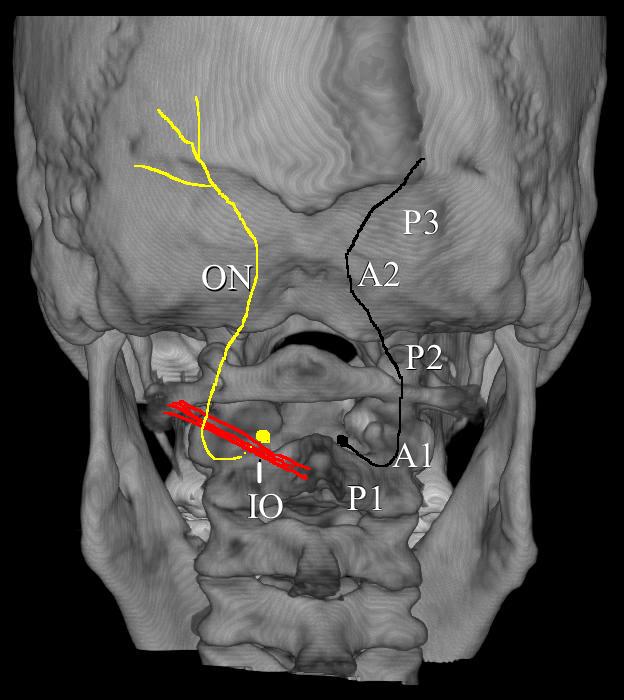
Figure 16. The path of the occipital nerve. IO = inferior oblique; P1 = Part 1 of the occipital nerve; P2 = Part 2 of the occipital nerve; P3 = Part 3 of the occipital nerve; A1 = site of entrapment of inferior oblique; A2 = site of entrapment by trapezius muscle. (Image: Andrea Trescot, MD)
The GON could therefore be considered to have three parts (Part 1, Part 2, and Part 3) as well as two bends (A1 and A2, where entrapments occur)45 (Figure 16). A cutaneous branch of the suboccipital nerve (the C1 dorsal ramus) will occasionally join the GON as it accompanies the occipital artery. The GON frequently connects with the lesser occipital nerve (Figure 15), which arises from the cervical plexus (formed by the upper four ventral cervical rami). The GON continues to ascend to innervate the skin along the posterior portion of the occiput to the vertex, portions of the ear, and parotid glands.
Shimizu et al.46 looked at the suboccipital region of 24 cadaver heads; the occipital nerve was found to cross over the artery, with the nerve consistently indented by the artery but without histologic evidence of mechanical damage. Natsis el al.47, based on the dissections of 40 cadavers, noted that GON was noted to get wider as it moved toward the periphery, and, in 3 cases, the nerve split into 2 branches before piercing the trapezius. The GON and lesser occipital nerves were reunited at the level of the occiput in 80% of the specimens. Becser, Bovim, and Sjaastad48 dissected 10 cadavers and found that twelve of the 20 GONs wrapped around the occipital artery.
Dash et al.49 looked at 19 cadavers and noted that all of the GONs identified were found to pierce the semispinalis capitis. On the other hand, in the 20 cadavers that Bovim et al.50 dissected (none of whom had a history of headaches), 10 specimens had the occipital nerve piercing the trapezius muscle on one side but not the other. They also identified 11 nerves with macroscopic signs of possible nerve compression from fibrotic tissue, with 6 compressions accompanied by a “kink” of more than 90°. Additionally, three of the 40 occipital nerves penetrated the inferior oblique. They also noted a marked variation of the amount of venous plexus at the level of C2; 50% of the cases had the C2 nerve root surrounded by a marked vascular network. They noted a significant variation in the path of the occipital nerve, but they also remarked that, for many of the dissections, there was a significant difference noted within the 2 sides of the same individual.
Entrapment
There are several areas of entrapment of the occipital nerve: (A) where the greater occipital nerve emerges from the C2 DRG between the atlas and axis, (B) between the inferior oblique and the semispinalis capitus muscles, (C) where the nerve pierces the semispinalis capitis, and (D) where the nerve exits from the aponeurosis of the trapezius. Because the GON pierces the nuchal fascia at the base of the skull, it is prone to trauma from flexion/tension injuries, which can lead to entrapment by a spasm of the trapezius muscle. Repetitive neck contractions secondary to work, recreational activities, and many other activities can cause entrapment and/or scarring of the GON. The trapezius or sternocleidomastoid muscles can cause entrapment secondary to myofascial spasms. Head forward positions can entrap the GON at the level of the inferior oblique. Medical conditions such as osteochondroma (benign tumors of the bone),51 arterial compression of the C2 nerve root by a tortuous vertebral artery, herpes zoster,52 osteoarthritis or rheumatoid arthritis (especially at C1-C2),53,54 or trauma such as blows to the back of the head or posterior fossa surgery, and many others contribute or are the root cause of entrapment or are the contributing factors causing pain. However, as the ganglion of this nerve interconnects with the trigeminal nerve in the brainstem, pain may be referred to any branch of the trigeminal nerve. The best way to diagnose an occipital nerve entrapment is by physical examination and injection.
Physical Examination
For the physical Examination of the greater occipital nerve, the patient should be positioned sitting with the neck slightly flexed, with the examiner supporting the forehead with the non-examining hand (Figure 17A). With the examining hand, place the middle finger at the midline base of the head to identify the site of the foramen magnum (Figure 17B). The index finger is placed at the conjoined tendon attachment (Figure 17C). The thumb will then palpate the area just lateral to the conjoined tendon, approximately 3cm inferior and 1.5cm lateral to the occipital inion (Figure 17D). If an area of paresthesia is created, reproducing the patient’s pain and symptomatology, the greater occipital nerve is likely the source.
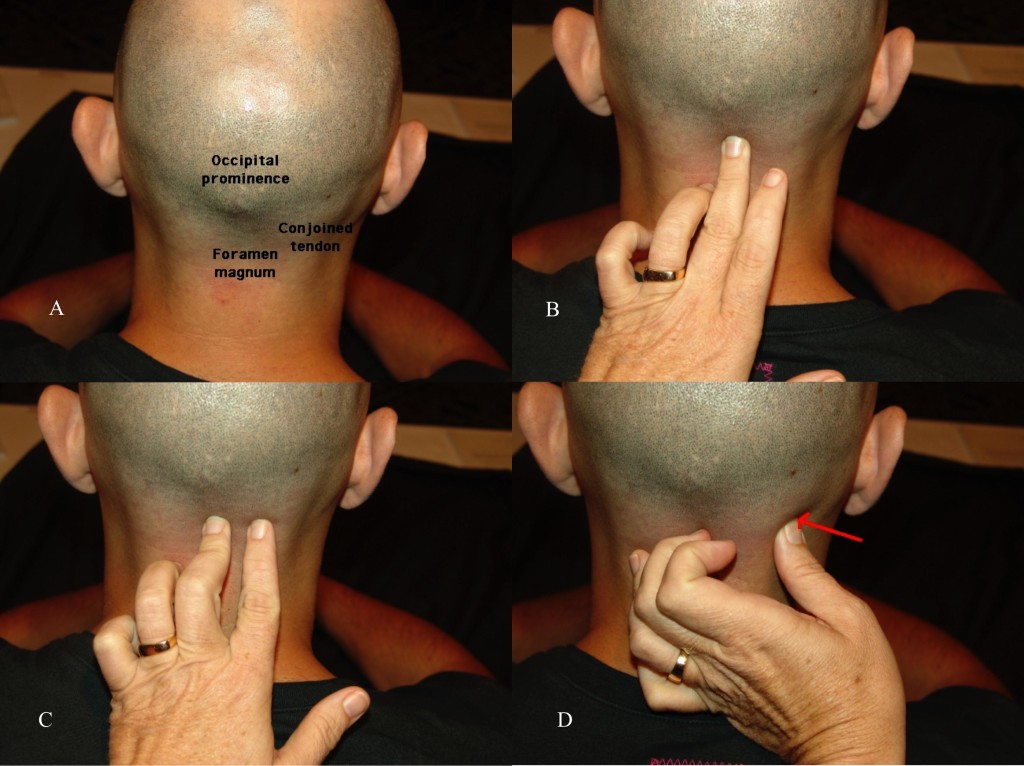
Figure 17 A, B, C, and D. Physical examination of the greater occipital nerve. (Images: Andrea Trescot, MD)
Ultrasound-Guided Technique
There are several sites for injection of the GON under US. Shim et al. (55) used US-guided injections at the occipital ridge (site 1 on Figure 18) to treat occipital headaches; they measured the distance from the “external occipital prominence” (the inion) to the occipital nerve and artery, and then looked at the results of landmark-guided versus US-guided injections. They found a greater improvement in pain scores for the US patients at 4 weeks (blind 3.8 ± 0.3 vs. US 2.3 ± 0.2). Na and colleagues56 did a similar study with 26 patients using flow Doppler versus blind injections at the nuchal ridge. All of the injections resulted in at least “partial” hypoesthesia; only 30.8% of the blind injections had “complete” anesthesia, while 76.9% of the Doppler-guided injections had “complete” anesthesia. Palamar et al. randomized 23 migraine patients to injections of 1.5cc of local anesthetic (0.5% bupivacaine) or saline under US guidance at the occipital ridge; all actively treated patients noted a significant decrease in headache symptoms.57 Each ON was found to be medial to the occipital artery.
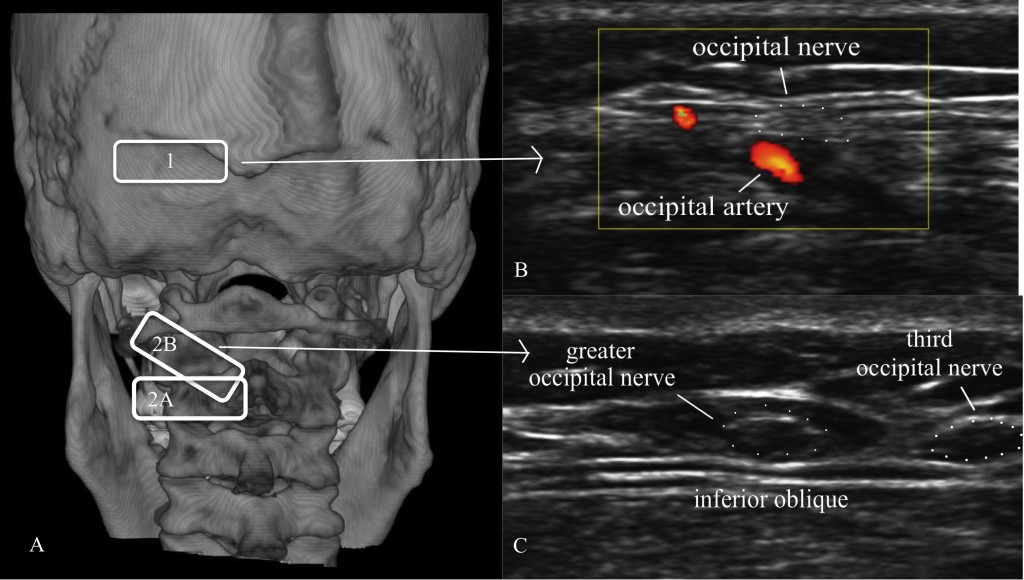
Figure 18. A = Location of ultrasound transducer: 1 = standard occipital nerve ultrasound site; 2 = US approach with (2A) being the initial probe placement and (2B) being the final probe placement. B = Distal US occipital nerve and artery. C = Proximal US of greater and third occipital nerve. (Images: Andrea Trescot, MD - modified from Greher et al. [48])
Greher et al.58 described an anatomic study on cadavers using ultrasound to compare the traditional injection site (site 1 on Figure 18) at the nuchal ridge with a more proximal technique (site 2 on Figure 18A). They identified the bifid spinous process of C2 with the probe in a horizontal position (site 2A on Figure 18A); the probe was then rotated (site 2B on Figure 18A) to visualize the inferior oblique muscle and the GON, which appears as a hypoechoic oval on top of the muscle (Figure 18C). Using 0.1cc injections of dye, they used ultrasound to identify the GON at both sites bilaterally in 10 cadavers. They reported successful injection of the GON distally in 16 of 20 dissections, while all 20 of the proximal GON were stained with dye (i.e., successful).
CONCLUSION
Aggressive treatment of headaches and “migraines” can be gratifying and technically satisfying for the interventional pain physician with the knowledge and skill to provide injection therapy to patients with these pain problems. US imaging and US-directed injections of “epicranial” structures can increase the likelihood of success.
Conflict of interest: None declared by the author
REFERENCES
Medical Director, Pain and Headache Center, St. Augustine, FL 32080 (USA)
Correspondence: Andrea Trescot, MD, 4 Oceanside Circle, St. Augustine, FL 32080 (USA); Cell: 904-806-6166; E-mail: DrTrescot@gmail.com
ABSTRACT
Headaches are a common pain complaint, affecting millions of people in the US alone. These pains have been attributed to intracranial processes, such as dilated blood vessels and the trigeminal ganglion, and therefore only amenable to medication therapies. However, many of these headaches, including “migraines”, may be caused by extracranial conditions that can be diagnosed and treated with interventional pain techniques. Using “pattern recognition”, many headache etiologies can be quickly identified, and then treated with ultrasound-guided injections that diagnose and treat the underlying condition. The topics in this article include the diagnosis and ultrasound-guided treatment of supraorbital neuralgia, auriculotemporal neuralgia, and greater occipital neuralgia, and their role in headache management.
Key words: Headache; Migraine; Supraorbital neuralgia; Auriculotemporal neuralgia; Occipital neuralgia; Ultrasound-guided injections
Citation: Trescot A. Ultrasound for evaluation and treatment of headaches. Anaesth Pain & Intensive Care 2017;21(2):241-253
Received: 15 Aug 2015, Reviewed: 2 Jun 2016, Accepted: 28 Jun 2016
INTRODUCTION
Headaches are one of the most prevalent neurologic disorders. They affect 28 million people in the US1 and account for millions of dollars in medical costs, lost labor costs, and associated burdens on our society. As with many medical events, headaches are complex, and our understanding of them is an evolving science. Although often seen as the primary pathology, headaches are fundamentally a symptom and have numerous proven origins and causes. There are many types of headaches, and no two headaches are the same. Despite the individual variations, one can see distinctive patterns of pain that can be easily recognized over and over again. Using pattern recognition, many headaches can be quickly diagnosed, treated, and, with proper care, put to an end entirely. Here, I propose that headaches, including migraines, are not always an isolated intracranial phenomenon. Rather, they can be an interaction between the intracranial components of the brain and the extracranial nerves. In 2003, Pareja et al2 proposed the term “epicrania” for headaches triggered by extracranial causes.
Plastic surgeons at the beginning of this century noted the relief of migraines with both corrugator muscle resection3 and injection of botulinum toxin,4 suggesting a peripheral headache trigger. Headache specialists repeatedly see patients with severe disabling migrainous headaches after a head or neck injury, and these headaches respond only modestly to migraine-specific pain medications. These headaches may have an extracranial origin, and the pain is likely a result of a peripheral nerve irritation and entrapment in the head or neck that was sustained during the injury. Moreover, this peripheral nerve irritation may have secondarily activated the migraine centers in the brain to bring about the associated migrainous symptoms. The treatment, therefore, would be to primarily inhibit the nerve irritation utilizing interventional pain techniques and thus turn off the pain origin, which subsequently turns off the activated migraine centers.
Although these nerves have been evaluated and injected in the past using landmarks and occasionally fluoroscopic images, ultrasound may offer some unique advantages, especially since most of these nerves travel with arteries, perhaps contributing to the complaints of “throbbing” pain.
EXTRA-CRANIAL CAUSES OF “MIGRAINES”, HEADACHES & FACE PAIN
Collectively, headache patterns have provided practitioners and researchers with basic guidelines to further understand and treat this often-debilitating condition. One of the commonly followed classification schemes for headaches is the International Classification of Headache Disorders (ICHD).5 This classification has defined many types of head pain from the viewpoint that headaches are either primary or secondary. The ICHD focuses on the categorization and description of syndromes of headache patterns. While thorough and extremely useful, this lexicon only generally describes certain peripheral nerve contributions to headaches as “Other Terminal Branch Neuralgias.” The ICHD does little to delineate the specific pain patterns of most of the individual extracranial nerve pathologies in the head and neck. The study of the origins of pain, as a function of extracranial peripheral nerve entrapments and their dysfunction, reveals a great deal of overlap between many of the ICHD-defined headaches and the nerve entrapments potentially causing these pain patterns.6 The study of headaches, as a subspecialty in the field of neurology, incorporates evidence from closely associated disciplines such as pain management, which become an invaluable tool in the growing repertoire available to headache practitioners.
SUPRAORBITAL NERVE
Supraorbital neuralgia (SN), first described by Beyer in 19497 is a cause of extracranial headaches, with a reported incidence of 4%.8 SN is defined by the International Headache Society (IHS)9 as a localized headache in the forehead region with the following criteria: paroxysmal or constant pain in the region supplied by the supraorbital nerve.2 SN has multiple etiologies and can sometimes be confused with migraine-type headaches, cluster headaches, or sinusitis. It presents as supraorbital, retro-orbital, and/or forehead pain, unilateral or bilateral, sharp and/or throbbing (Figure1). It may be spontaneous or the result of palpation of the supraorbital area. Occasional patients may complain of blurred vision, nausea, and photophobia, thereby confusing the diagnosis.10 Triptans can help but usually only temporarily, presumably by vasoconstricting the blood vessels travelling with the nerve (Figure 2), thereby decreasing the entrapment. Symptoms include continuous or intermittent unilateral pain above the eye that occasionally radiates distally along the SON to the vertex of the skull.

Figure 1: Patient pain complaint from supraorbital nerve entrapment. (Image: Andrea Trescot, MD)
The SON can also serve as a trigeminal “trigger zone” for tic douloureux. The symptoms may worsen with time, especially if they are associated with progressive scarring around the nerve. Headache symptoms are often exacerbated with excessive squinting or frowning (when the orbicularis oculi entraps the nerve), direct pressure (such as with swimming goggles), the head held lower than the heart (because of increased blood flow), or fluid retention associated with pre-menses and excessive salt consumption (which causes swelling of the nerve in its canal). All these factors can lead to further compression of the nerve.
Like hemicrania continua and cluster headaches, the headache from supraorbital nerve entrapment can have associated ipsilateral conjunctival injection, lacrimation, nasal congestion, rhinorrhea, and frequent ipsilateral conjunctival injection, lacrimation, nasal congestion, rhinorrhea, and frequent “attacks.” In fact, hemicrania continua has been successfully treated with supraorbital and supratrochlear nerve injections.11 And as with migraines, there may be associated photophobia, phonophobia, nausea, and emesis. The pain is almost always unilateral and can be severe enough to lead to suicidal thoughts.12
Anatomy
The SON is one of five peripheral branches of the ophthalmic division of the trigeminal nerve (cranial nerve V). The trigeminal ganglion (TG) gives rise to three divisions, ophthalmic (V1), maxillary (V2), and mandibular (V3). While the V2 and V3 divisions branch downward, the V1 division branches superior and medially from the TG until it enters the orbit posteriorly via the superior orbital fissure. The nerve then divides into 3, continuing on as the lacrimal nerve, the nasociliary nerve, and the frontal nerve. The frontal nerve then splits into the supraorbital nerve (SON), which exits the orbit with the supraorbital artery via the supraorbital notch (Figure 2), and the supratrochlear nerve (STN), which exits with the supratrochlear artery via the supratrochlear groove or notch (Figure 2). and runs medially in a small groove at the junction of the ocular ridge and the nares. The SON then branches into a medial and lateral branch over the forehead (Figure 3).

Figure 2: Supraorbital nerve and artery dissected

Figure 3: Facial arteries; arrow identifies the supraorbital artery (Image from Quain J, Wilson WJE. The Vessels of the Human Body, 1837)
Separate exits for the medial and lateral SON branches were observed in eight of the 28 nerves (29%) examined by Anderson et al.13 The medial branch continues superiorly up to the vertex of the skull, while the lateral branch moves superior-laterally; both branches supply sensory fibers to the forehead, upper eyelid, and anterior scalp, up to the lambdoidal suture, and both were associated with arteries (often intimately entwined). Janis et al. found that 73% of the specimens they examined had the SON passing through the corrugator muscle.14
The supraorbital notch may be covered by a small ligament (creating a foramen), which can become thickened or calcified (Figure 4).

Figure 4: 3D image of the skull. Arrows show supraorbital notch. A = obliterated foramen, B = supraorbital notch. C = supratrochlear groove. (Image: Andrea Trescot, MD)
Fallucco and colleagues called this ligament-covered foramen a “miniature carpal tunnel”.15). In dissections done by Anderson et al.,13 20 of 28 nerves (71%) exited through foramina (with bony or connective tissue bridges) instead of notches. Fallucco et al.15 dissected 50 SONs and found that 43 of the 50 (86%) had fascial bands across the notch. These were further divided into “simple” bands (22 of 43 or 51.2%), “partial bony” (13 of 43 or 30.2%), and ‘septums’, which could be horizontal (4 of 43 or 9.3%) or vertical (4 of 43 or 9.3%). There was a high degree of variability, and the authors noted that the anatomic difference and variable diameters could account for unilateral migraine symptoms.
Entrapment
Entrapment occurs primarily at the supraorbital notch as the nerve leaves the orbit, passing through the supraorbital notch, the orbicularis oculi muscle, the glabellar muscle, and the corrugator muscle, and it can be exacerbated by frowning and squinting (perhaps the reason that some “migraines” respond to use of eyeglasses or botulinum toxin). Tight fitting goggles16 or an anesthesia mask17 can compress the nerve. There can also be trauma to the nerve from surgery in the frontal region,17 This headache will often worsen with menses or increased salt intake, perhaps by causing swelling of the nerve in its canal. In addition, there can be entrapment by scarring17 as well as by thickening or calcification of the supraorbital ligament.
Physical Examination
A thorough history should first be obtained to identify any previous trauma or inciting events that could lead to SN. This is then followed by a visual inspection and physical examination of the forehead, eyebrow, nasal region, and supraorbital foramen. There will be tenderness to palpation over the supraorbital foramen with possible radiation of symptoms along the nerve distribution of the affected side. Support the head with the non-examining hand, then palpate over the orbital rim, feeling for the supraorbital notch (Figure 5). Parasthesias should replicate the pain complaints, and, with careful palpation, the examiner should be able to identify the slim, string-like vertical SON structure. Move the thumb medially to feel the supratrochlear groove.

Figure 5: Physical examination of the supraorbital nerve. (Image: Andrea Trescot, MD)
In 2005, Sjaastad and colleagues reported on the supraorbital examination of 1,828 of the inhabitants of Vågå, Norway.12 Only 24 persons (1.3%) did not have a palpable SON; 98% of the individuals had a palpable SON, and 5.4% of the inhabitants (106 of the 1,828 people examined) had increased tenderness over the supraorbital notch. Twelve of these had a clinical presentation of SN; 10 of the 12 had a history of forehead trauma.
Ultrasound Examination
Using ultrasound (US) to block the supraorbital nerve can be beneficial over the landmark-guided or fluoroscopic approaches. The linear probe is placed horizontally across the supraorbital notch (Figure 6). With direct visualization of the supraorbital foramen, proper needle placement can be achieved as well as avoiding inadvertent needle placement into the foramen. In a recent cadaveric study, Spinner and colleagues18 demonstrated that ultrasound-guided blocks of the supraorbital and infraorbital nerves had greater accuracy than conventional landmark-based techniques. The results of the study showed that the US accuracy rate was 100% (18 of 18) for the in-plane approach and 94% (17 of 18) for the out-of-plane approach. Thirty-five injections were considered accurate (97%) with overflow, and one injection was inaccurate. The study concluded that ultrasound-guided injections had a higher degree of accuracy versus the standard techniques used today. The US technique would be performed the same as the conventional landmark-guided technique with improved visualization of the target.

Figure 6: Ultrasound image of the supraorbital nerve. (Ultrasound Image: David Spinner, MD; composite created by Andrea Trescot, MD)
AURICULOTEMPORAL NEURALGIA
Temple headaches may be due to entrapment of the auriculotemporal nerve (ATN), a third-division branch of the trigeminal nerve. Other areas of pain when this nerve is entrapped may include pain in the auricular area and the temporomandibular joint. This is a common headache site (just visualize all the headache patients rubbing or pressing their temples for relief) shared with migraine headaches, and it may often be misdiagnosed as an intracranial migraine. There are two distinct clinical presentations of ATN nerve dysfunction, and a variety of names, including auriculotemporal neuralgia, auriculotemporal syndrome, Frey syndrome, Baillarger syndrome, Dupuy syndrome, salivosudoriparous syndrome, and gustatory sweating syndrome.
Clinical Presentation
Entrapment of the ATN is probably the most common of the trigeminal headaches. Calandre et al.19 evaluated specific areas in the scalp that could be manually palpated to elicit a headache; 42.6% of these “trigger points” were found in the temple region and 33.4% in the suboccipital region. However, ATN entrapment is rarely diagnosed and represented only 0.4% of the referrals to a tertiary headache outpatient clinic.20
There are two main presentations of irritation of this nerve: Auriculotemporal Neuralgia (ATn) and the rarer Auriculotemporal Syndrome (ATS), both of which can have overlapping presentations, as well as multiple etiologies.
ATn typically presents as paroxysmal attacks of pain in the preauricular and temple regions as well as the retro-orbital region and the teeth.21 The patient with ATn headaches due to ATN entrapment will often awaken at 3-4am with a headache in the auricular area that radiates into the temple (Figure 7) and the retro-orbital as well as the occipital region.

Figure 7: Patient’s description of pain from auriculotemporal neuralgia. (Image: Andrea Trescot, MD)
The headache may be unilateral22 or bilateral, and may have associated ear, parotid, and jaw pain or numbness.20 The headache is often throbbing in nature, possibly due to its proximity to the temporal artery. There is often increased pain with talking, chewing, and menses.23 The intensity of the pain is moderate to severe, and the quality is usually constant, though at times there may be paroxysms of sharp, jabbing, lancinating pain or a throbbing, pounding pain. The pain may be triggered by trauma to the jaw or temple, as well as by surgery, exacerbated by palpation over the preauricular area or by chewing, and can be associated with severe nausea and vomiting. The pain characteristics and accompanying nausea and vomiting meet the International Headache Society criteria9 for a “migraine”, though there is no specific listing for ATn. The early morning headache appears to be related to bruxism or nocturnal jaw clenching. Because there is a physical connection with the facial nerve (see Anatomy section), there can also be pain in the muscles of facial expression with ATN entrapment.
In 2005, Speciali and Gonçalves 20 evaluated six patients with ATn proven by diagnostic nerve blocks (see Injection techniques section). All were females with unilateral pain, suffering with symptoms from 1 month to 20 years, and all of the patients had pain with palpation of the preauricular area just above the tragus. Pain was primarily around the ear, radiating to the head of the mandible and temple in all of the patients. One patient had knife-like pain triggered by gustatory stimuli (see Frey’s syndrome below). In 4 patients, the pain radiated to the occipital region, but there was no relief from occipital nerve blocks (see below). There were complaints of facial tingling or parasthesias in 4 patients, but, notably, temporomandibular joint (TMJ) pathology was absent in all of the patients. All of the patients had complete or near-complete relief with ATN injections.
Murayama et al.21 described a patient with left ear pain and ipsilateral maxillary second molar pain. The dental examination was normal, and the tooth and ear pain resolved with a local anesthetic and steroid injection of the ATN, with remission still present 6 months later.
The Auriculotemporal Syndrome, also known as Frey syndrome, was described by Frey in 192324 as a constellation of symptoms including unilateral hyperhidrosis and flushing of the cheek and ear that occurs when eating or drinking anything that stimulates the parotid gland to produce saliva (Figure 8). Pain is uncommon, but there may be sensory changes in the ATN distribution, and there may be a concomitant trigeminal neuralgia. Also known as Baillarger syndrome, Dupuy syndrome, salivosudoriparous syndrome, and gustatory sweating syndrome, these symptoms usually occur 2 to 13 months after surgery, open trauma, or infection of the parotid gland, though it has also be described after dislocation of the mandibular condyle.25 The proposed cause is an improper regeneration of the sympathetic and parasympathetic nerves of the parotid gland, and the syndrome occurs in about 5% of surgical patients, even with careful technique. The severity of symptoms associated with Frey syndrome can range from mild to debilitating.

Figure 8: Frey syndrome. Note the well-healed parotid scar and the facial flushing with eating.
Gordon and Fiddian26 reviewed the records of 71 patients who had parotid surgery; 17 patients had “noticeable” Frey syndrome. Fourteen patients had positive Minors’ test (iodine/starch), with the greater auricular nerve involved in 6 cases, the ATN in 4 cases, and both in 2 cases (with 2 inconclusive tests). Choi et al.27 evaluated 59 patients after parotidectomy, using subjective symptoms, Minor’s starch iodine test, and thermography, and found a “good” correlation between the 3 indicators. Pain triggered by taste stimuli can be a significant component of Frey’s syndrome; Scrivani et al.28 described 6 patients with recurrent, episodic shocking facial pain triggered even by the smell of food, which occurred days to weeks after head and neck surgery.
Anatomy
The ATN is a branch of the third division (mandibular) of the trigeminal ganglion. The mandibular nerve exits the cranium through the foramen ovale and then immediately divides into an anterior and posterior division, providing sensation to the TMJ, tragus, external auditory canal, parotid, base of the auricle, and skin of temporal region (Figure 9).

Figure 9: Nerves of the face. (Image: Andrea Trescot, MD)
The anterior division travels between the roof of the infratemporal fossa and the lateral pterygoid muscle (LPM) and is composed of the anterior deep temporal nerve, the posterior deep temporal nerve, and the masseteric nerves. The posterior division consists of the lingual nerve, inferior alveolar nerve, and ATN, descending medial to the LPM.29 Because the ATN has a long and tortuous course, it is at risk for irritation and entrapment. The ATN arises by two roots, which travel on either side of the ascending middle meningeal artery and then join together to form a short trunk. It leaves the foramen ovale, and runs beneath the pterygoideus externus to the medial side of the neck of the mandible. It then turns upward with the superficial temporal artery, between the external ear and condyle of the mandible, under the parotid gland. After it leaves the gland, it ascends over the zygomatic arch, traveling in front of the temporal mandibular joint (providing the primary enervation of the joint as it goes by) to pierce the temporalis muscle. The ATN then divides into five small branches, including the nerve to the external auditory meatus, which innervates the skin of the meatus and the external tympanic membrane; the parotid branch nerves; the articular branches, which innervate the posterior part of the TMJ; and the superficial temporal nerve, which innervates the skin of the temporal region. The superficial temporal nerve communicates with the facial nerve, the zygomaticotemporal nerve (which is a branch of the maxillary nerve) and zygomatic branches of the facial nerve.
The ATN has been associated with migraine headaches. Chim et al.30 dissected the ATN in 20 cadavers and found 3 potential compression points along the path of the nerve. Point 1 and 2 corresponded to fascial bands, with point 1 being 13.1 ± 5.9mm anterior and 5 ± 7mm superior to the superior point of the external auditory meatus and point 2 found at 11.9 ± 6mm anterior and 17.2 ± 10.4mm superior to the most anteriosuperior portion of the external auditory meatus. The 3rd point represented the interaction of the ATN and the superficial temporal artery (Figure 10), found in 80% of the dissection. There were 3 types of interactions noted – the artery crossing over the nerve (62.5%), the nerve crossing over the artery (18.8%), or the artery wrapping helically around the nerve (18.8%).

Figure 10: Temple arteries; arrow identifies the temporal artery (Image from Quain J, Wilson WJE. The Vessels of the Human Body, 1837)
Andersen et al.13 dissected 10 heads and found the superficial ATN branch to be located between 8 and 20mm anterior to the root of the ear helix. Only 4 of the cadavers had a single branch anterior to the tragus; the rest had multiple branches. The temporal artery was always found deep to the ATN, and in 3 dissections the ATN connected with the temporal branch of the facial nerve. There was noted to be a connection between the ATN and lesser occipital nerve in 2 cases, and between the ATN and the greater occipital nerve in 4 cases.
Janis et al.1 dissected 50 cadaver temples and identified that the ATN was always found to lateral to the artery in the upper preauricular temple. In another 50 cadavers, Janis et al.31 dissected the periorbital and temporal regions, evaluating the path of the zygomaticotemporal branch of the ATN. In “exactly half” of the specimens, the nerve had no intramuscular path; in the other half of the cadavers, 11 had only a brief course through the muscle, but 14 had a long, tortuous path through the muscle, making entrapment likely.
Entrapment
Entrapment of the posterior division of the mandibular nerve is not uncommon,20 with one study showing entrapment in 6% of 52 cadavers, primarily at the level of the LPM.29 Another study of 10 cadaver dissections showed myositis, local ischemia, and inflammation of the LPM in patients with premortem complaints of pain in an ATN distribution.32 Spasm of the LPM or local changes within the muscle can entrap the nerve, causing numbness of the external ear and TMJ.29 The ATN can also be entrapped between the medial and lateral pterygoid muscles, causing facial numbness, mandibular pain, or headaches.33
The anatomic relationships between the ATN and the muscles of mastication, the TMJ, and the surrounding vascular structures set up the potential for entrapment, so that ATn can play a role in TMJ pathology, headaches, and external ear pain. Secretomotor fibers within the parotid gland may also be entrapped, leading to impairment of salivation (and therefore triggering Frey syndrome).
Janis et al.1 proposed an entrapment of the ATN by the superficial temporal artery, finding a “direct relationship” between the ATN and the superficial temporal artery in 34% of the cadavers studied, with the ATN wrapped helically around the superficial temporal nerve. Because of the ATN and facial nerve connection, entrapment of the nerve at this level can cause pain in the muscles of facial expression.29
In addition, the temporal artery entraps the zygomaticotemporal nerve (a branch of the maxillary nerve that exits through the zygomaticotemporal foramen and innervates a small area of the forehead and temporal region), mimicking an ATN.34
Physical Examination
Since there are several sites of entrapment, the physical examination must be able to separate each area of pathology. Examination should include the TMJ (placing the examining fingers over the joint as the patient opens and closes their mouth), the masseter (rolling the examining thumb horizontally as the patient clenches their jaw) and the zygomatic branch of the facial nerve (placing the examining thumb at the anterior condyle of the mandible, just under the zygoma). The examiner should also palpate of the temporalis muscle (looking for trigger point tenderness) and the superficial temporal artery (to evaluate for temporal arteritis).
The most clinically common site of entrapment for the ATN is in the temporal fossa; Trescot described finding the ATN by placing the index finger at the apex of an isosceles triangle created by resting (for the right-sided exam) the thumb on the tragus and the middle finger on the canthus (corner of the eye) (Figure 11).23

Figure 11: Auriculotemporal nerve examination. (Image: Andrea Trescot, MD)
Ultrasound Guided Injection
In 2007, Shankar and Brethauer35 described an US technique for the ATN injection. The US probe is placed transversely just above the TMJ, and color Doppler used to identify the superficial temporal artery; the nerve appears as a small hyperlucent bundle (Figure 12A). The probe is then rotated to perform a longitudinal scan to track the nerve cephalad (Figure12B). They used a 25g needle from an out-of-plane approach to deliver medication (2cc of 1% lidocaine and 10mg of Depo-Medrol®) around the nerve.

Fig 12: Ultrasound evaluation of the auriculotemporal nerve. A = horizontal evaluation; B = vertical evaluation. (Images: Andrea Trescot, MD)
Because of its tortuous path, its proximity to a significant artery (the temporal artery), and its frequent misdiagnosis, US is an excellent tool for the diagnosis and treatment of the ATN
OCCIPITAL NERVE
The greater occipital nerve (GON) entrapment commonly causes occipital and cervicogenic headaches, and can sometimes cause headaches with characteristics similar to migraine headaches. The anatomic recognition of multiple occipital nerves (the greater occipital, the lesser occipital, and the third occipital nerve), each with different patterns of pain, neurologic origin, and treatment modalities, has been facilitated by the use of precision diagnostic injections. This article will focus specifically on the GON. There are multiple names used for these nerve entrapments, including “migraine”, “tension headache”, “cervicogenic headaches”,36 and “occipital myalgia-neuralgia syndrome”.37 Sometimes the term “suboccipital injections” has been used to describe greater occipital nerve injections, but, for this article, I use “suboccipital” to mean the C1 nerve root or the high volume occipital decompression. Occipital neuralgia is also known as C2 neuralgia or Arnold’s neuralgia.
Clinical Presentation
Beruto et al.38 described occipital neuralgia (ON) in 1821 as a sharp, lightening-bolt pain radiating from the occiput to the vertex. The medical terms occipital neuralgia and cervicogenic headache describe a syndrome of neck and head pain primarily referring to the occiput, as well as the temporal area, forehead, and retro-orbital areas, that may arise some distance away, in the upper cervical spine. Greater occipital neuralgia characteristically presents as paroxysmal shooting, stabbing pain from the suboccipital region to the vertex (Figure 13). The cutaneous innervation of the posterior neck and occiput comes primarily from C1, C2, and C3, which provide overlapping dermatomes (Figure 14). C2 covers the occiput, neck, and submental regions, while the C3 dermatome can span from the clavicle to the mandible to behind and over the ear, pinna, and the angle of the mandible.39 Since the greater occipital nerve is made up of contributions from C1, C2, and C3, there can be a wide range of clinical presentations.

Figure 13. Pattern of greater occipital pain. (Image: Andrea Trescot, MD)

Figure 14. Pattern of posterior cervical and occipital pain. A = C2/3 facet; B = C3/4 facet; C = greater occipital nerve (GON); D = posterior auricular nerve (PAN); E = lesser occipital nerve (LON); F = third occipital nerve (TON). (Image: Andrea Trescot, MD)
As a subset of cervicogenic headaches (CGH), occipital neuralgia can cause pain and paresthesias to the posterior scalp, the periorbital, temporal and mandibular regions, the external ear and mastoid regions, as well as pain in the neck and shoulders. The first three cervical spinal nerve segments (C1-C3) that make up the occipital nerves share a relay-station in the brainstem that continues into the upper cervical spinal cord with the trigeminal cell bodies (the cervico-trigeminal complex). The pain of occipital neuralgia and cervicogenic headaches can therefore be referred to structures innervated by the branches of the trigeminal nerve, namely, the forehead, temples, and eyes.
There are three occipital nerves – the greater, the lesser, and the third occipital nerves. Their patterns of pain are overlapping, and so the reader is encouraged to review information on these nerves as well.
Unilateral ON, perhaps because of the proximity of the occipital artery (see below), can present with throbbing, unilateral headaches associated with photophobia, phonophobia, and nausea; symptoms meet the International Classification of Headache Disorders (ICHD) of a “migraine”.5 There may be a history of occipital pain, often radiating to the face, specifically described as “behind my eye” and “like an ice pick”. Occipital headaches may start as “tension headaches” in the upper cervical region, but then center at the base of the skull and produce throbbing, unilateral or bilateral pain, accompanied by nausea, photophobia, and phonophobia. Occipital neuralgia has a close relationship with cluster headaches, with several authors40-42 describing the use of occipital blocks to treat cluster headaches. Hemicrania continuum43 and “transformed migraines”44 have also responded to occipital nerve blocks.
Anatomy
The largest of the three occipital nerves, the GON arises from the posterior ramus of C2 that runs inferiorly between the arch of C1 (atlas) and the lamina of C2 (axis), lateral to the lateral atlantoaxial (AA) joint, deep to the inferior oblique muscle. The GON then curves up over the inferior oblique, between the inferior oblique and the semispinalis capitis muscles (Figure 15).

Figure 15: Anatomy of the occipital region, modified from an image from Bodies, The Exhibition, with permission. Note the connection of the greater and lesser occipital nerves. (Image: Andrea Trescot, MD)
A branch from C3 may join at this point, as the nerve ascends up the neck and over the dorsal surface of the rectus capitis to pierce the semispinalis capitis muscle, deep to the trapezius muscle. The GON then exits the neck through a muscular sling formed by the aponeurosis of the sternocleidomastoid muscle (SCM) and trapezius muscle at their attachment on the occipital bone (the conjoined tendon), where it is joined laterally by the occipital artery. At this point, the greater occipital nerve is immediately medial to the occipital artery and lateral to the inion or occipital prominence, lying in a palpable groove. This proximity to the artery may account for the throbbing sensation that often accompanies GON entrapment.

Figure 16. The path of the occipital nerve. IO = inferior oblique; P1 = Part 1 of the occipital nerve; P2 = Part 2 of the occipital nerve; P3 = Part 3 of the occipital nerve; A1 = site of entrapment of inferior oblique; A2 = site of entrapment by trapezius muscle. (Image: Andrea Trescot, MD)
The GON could therefore be considered to have three parts (Part 1, Part 2, and Part 3) as well as two bends (A1 and A2, where entrapments occur)45 (Figure 16). A cutaneous branch of the suboccipital nerve (the C1 dorsal ramus) will occasionally join the GON as it accompanies the occipital artery. The GON frequently connects with the lesser occipital nerve (Figure 15), which arises from the cervical plexus (formed by the upper four ventral cervical rami). The GON continues to ascend to innervate the skin along the posterior portion of the occiput to the vertex, portions of the ear, and parotid glands.
Shimizu et al.46 looked at the suboccipital region of 24 cadaver heads; the occipital nerve was found to cross over the artery, with the nerve consistently indented by the artery but without histologic evidence of mechanical damage. Natsis el al.47, based on the dissections of 40 cadavers, noted that GON was noted to get wider as it moved toward the periphery, and, in 3 cases, the nerve split into 2 branches before piercing the trapezius. The GON and lesser occipital nerves were reunited at the level of the occiput in 80% of the specimens. Becser, Bovim, and Sjaastad48 dissected 10 cadavers and found that twelve of the 20 GONs wrapped around the occipital artery.
Dash et al.49 looked at 19 cadavers and noted that all of the GONs identified were found to pierce the semispinalis capitis. On the other hand, in the 20 cadavers that Bovim et al.50 dissected (none of whom had a history of headaches), 10 specimens had the occipital nerve piercing the trapezius muscle on one side but not the other. They also identified 11 nerves with macroscopic signs of possible nerve compression from fibrotic tissue, with 6 compressions accompanied by a “kink” of more than 90°. Additionally, three of the 40 occipital nerves penetrated the inferior oblique. They also noted a marked variation of the amount of venous plexus at the level of C2; 50% of the cases had the C2 nerve root surrounded by a marked vascular network. They noted a significant variation in the path of the occipital nerve, but they also remarked that, for many of the dissections, there was a significant difference noted within the 2 sides of the same individual.
Entrapment
There are several areas of entrapment of the occipital nerve: (A) where the greater occipital nerve emerges from the C2 DRG between the atlas and axis, (B) between the inferior oblique and the semispinalis capitus muscles, (C) where the nerve pierces the semispinalis capitis, and (D) where the nerve exits from the aponeurosis of the trapezius. Because the GON pierces the nuchal fascia at the base of the skull, it is prone to trauma from flexion/tension injuries, which can lead to entrapment by a spasm of the trapezius muscle. Repetitive neck contractions secondary to work, recreational activities, and many other activities can cause entrapment and/or scarring of the GON. The trapezius or sternocleidomastoid muscles can cause entrapment secondary to myofascial spasms. Head forward positions can entrap the GON at the level of the inferior oblique. Medical conditions such as osteochondroma (benign tumors of the bone),51 arterial compression of the C2 nerve root by a tortuous vertebral artery, herpes zoster,52 osteoarthritis or rheumatoid arthritis (especially at C1-C2),53,54 or trauma such as blows to the back of the head or posterior fossa surgery, and many others contribute or are the root cause of entrapment or are the contributing factors causing pain. However, as the ganglion of this nerve interconnects with the trigeminal nerve in the brainstem, pain may be referred to any branch of the trigeminal nerve. The best way to diagnose an occipital nerve entrapment is by physical examination and injection.
Physical Examination
For the physical Examination of the greater occipital nerve, the patient should be positioned sitting with the neck slightly flexed, with the examiner supporting the forehead with the non-examining hand (Figure 17A). With the examining hand, place the middle finger at the midline base of the head to identify the site of the foramen magnum (Figure 17B). The index finger is placed at the conjoined tendon attachment (Figure 17C). The thumb will then palpate the area just lateral to the conjoined tendon, approximately 3cm inferior and 1.5cm lateral to the occipital inion (Figure 17D). If an area of paresthesia is created, reproducing the patient’s pain and symptomatology, the greater occipital nerve is likely the source.

Figure 17 A, B, C, and D. Physical examination of the greater occipital nerve. (Images: Andrea Trescot, MD)
Ultrasound-Guided Technique
There are several sites for injection of the GON under US. Shim et al. (55) used US-guided injections at the occipital ridge (site 1 on Figure 18) to treat occipital headaches; they measured the distance from the “external occipital prominence” (the inion) to the occipital nerve and artery, and then looked at the results of landmark-guided versus US-guided injections. They found a greater improvement in pain scores for the US patients at 4 weeks (blind 3.8 ± 0.3 vs. US 2.3 ± 0.2). Na and colleagues56 did a similar study with 26 patients using flow Doppler versus blind injections at the nuchal ridge. All of the injections resulted in at least “partial” hypoesthesia; only 30.8% of the blind injections had “complete” anesthesia, while 76.9% of the Doppler-guided injections had “complete” anesthesia. Palamar et al. randomized 23 migraine patients to injections of 1.5cc of local anesthetic (0.5% bupivacaine) or saline under US guidance at the occipital ridge; all actively treated patients noted a significant decrease in headache symptoms.57 Each ON was found to be medial to the occipital artery.

Figure 18. A = Location of ultrasound transducer: 1 = standard occipital nerve ultrasound site; 2 = US approach with (2A) being the initial probe placement and (2B) being the final probe placement. B = Distal US occipital nerve and artery. C = Proximal US of greater and third occipital nerve. (Images: Andrea Trescot, MD - modified from Greher et al. [48])
Greher et al.58 described an anatomic study on cadavers using ultrasound to compare the traditional injection site (site 1 on Figure 18) at the nuchal ridge with a more proximal technique (site 2 on Figure 18A). They identified the bifid spinous process of C2 with the probe in a horizontal position (site 2A on Figure 18A); the probe was then rotated (site 2B on Figure 18A) to visualize the inferior oblique muscle and the GON, which appears as a hypoechoic oval on top of the muscle (Figure 18C). Using 0.1cc injections of dye, they used ultrasound to identify the GON at both sites bilaterally in 10 cadavers. They reported successful injection of the GON distally in 16 of 20 dissections, while all 20 of the proximal GON were stained with dye (i.e., successful).
CONCLUSION
Aggressive treatment of headaches and “migraines” can be gratifying and technically satisfying for the interventional pain physician with the knowledge and skill to provide injection therapy to patients with these pain problems. US imaging and US-directed injections of “epicranial” structures can increase the likelihood of success.
Conflict of interest: None declared by the author
REFERENCES
- Janis JE, Hatef DA, Ducic I, Ahmad J, Wong C, Hoxworth RE, et al. Anatomy of the auriculotemporal nerve: variations in its relationship to the superficial temporal artery and implications for the treatment of migraine headaches. Plast Reconstr Surg. 2010;125(5):1422-8. doi: 10.1097/PRS.0b013e3181d4fb05. [PubMed]
- Evans RW, Pareja JA. Expert opinion. Supraorbital neuralgia. Headache. 2009;49(2):278-81. [PubMed] doi: 10.1111/j.1526-4610.2008.001328.x.
- Guyuron B, Varghai A, Michelow BJ, Thomas T, Davis J. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106(2):429-34; discussion 435-7. [PubMed]
- Gobel H, Heinze A, Heinze-Kuhn K, Jost WH. Evidence-based medicine: botulinum toxin A in migraine and tension-type headache. J Neurol. 2001;248 Suppl 1:34-8. [PubMed]
- The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. doi: 10.1177/0333102413485658.[PubMed]
- Dalkara T, Zervas NT, Moskowitz MA. From spreading depression to the trigeminovascular system. Neurol Sci. 2006;27 Suppl 2:S86-90. [PubMed]
- Beyer TE. Idiopathic supraorbital neuralgia. Laryngoscope. 1949;59(11):1273-5. [PubMed]
- Agrawal SM, Kambalimath DH. Trigeminal neuralgia involving supraorbital and infraorbital nerves. Natl J Maxillofac Surg. 2010;1(2):179-82. doi: 10.4103/0975-5950.79226. [PubMed] [Free full text]
- The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9-160. [PubMed]
- Trescot AM. Cryoanalgesia in interventional pain management. Pain Physician. 2003;6(3):345-60. [PubMed] [Free full text]
- Guerrero AL, Herrero-Velazquez S, Penas ML, Mulero P, Pedraza MI, Cortijo E, et al. Peripheral nerve blocks: a therapeutic alternative for hemicrania continua. Cephalalgia. 2012 Apr;32(6):505-8. doi: 10.1177/0333102412439800. [PubMed] [Free full text]
- Sjaastad O, Petersen HC, Bakketeig LS. Supraorbital neuralgia. Vaga study of headache epidemiology. Cephalalgia. 2005;25(4):296-304. [PubMed] [Free full text]
- Andersen NB, Bovim G, Sjaastad O. The frontotemporal peripheral nerves. Topographic variations of the supraorbital, supratrochlear and auriculotemporal nerves and their possible clinical significance. Surg Radiol Anat. 2001;23(2):97-104. [PubMed]
- Janis JE, Ghavami A, Lemmon JA, Leedy JE, Guyuron B. The anatomy of the corrugator supercilii muscle: part II. Supraorbital nerve branching patterns. Plast Reconstr Surg. 2008 Jan;121(1):233-40. doi: 10.1097/01.prs.0000299260.04932.38. [PubMed]
- Fallucco M, Janis JE, Hagan RR. The anatomical morphology of the supraorbital notch: clinical relevance to the surgical treatment of migraine headaches. Plast Reconstr Surg. 2012 Dec;130(6):1227-33. doi: 10.1097/PRS.0b013e31826d9c8d. [PubMed]
- O'Brien JC Jr. Swimmer's headache, or supraorbital neuralgia. Proc (Bayl Univ Med Cent). 2004;17(4):418-9. [PubMed] [Free full text]
- Klein DS, Schmidt RE, Jr. Chronic headache resulting from postoperative supraorbital neuralgia. Anesth Analg. 1991;73(4):490-1. [PubMed]
- Spinner D, Kirschner JS. Accuracy of ultrasound-guided superficial trigeminal nerve blocks using methylene blue in cadavers. Pain Med. 2012 Nov;13(11):1469-73. doi: 10.1111/j.1526-4637.2012.01480.x. [PubMed]
- Calandre EP, Hidalgo J, Garcia-Leiva JM, Rico-Villademoros F. Trigger point evaluation in migraine patients: an indication of peripheral sensitization linked to migraine predisposition? European journal of neurology : the official journal of the European Federation of Neurological Societies. 2006;13(3):244-9. [PubMed]
- Speciali JG, Gonçalves DA. Auriculotemporal neuralgia. Curr Pain Head Rep. 2005;9:277-80. [PubMed]
- Murayama RA, Stuginski-Barbosa J, Moraes NP, Speciali JG. Toothache referred from auriculotemporal neuralgia: case report. Int Endod J. 2009 Sep;42(9):845-51. doi: 10.1111/j.1365-2591.2009.01599.x. [PubMed]
- Damarjian E. Auriculo-temporal neuralgia--an original diagnostic and therapeutic approach. R I Med J. 1970;53(2):100-1. [PubMed]
- Trescot AM. Headache: Is it a migraine? Think again. Tech Reg Anesth Pain Manag. 2005;9:68-72.
- Frey L. Le syndrome du nerf auriculo-temporal. Rev Neurol (Paris). 1923;2:97-104.
- Kamath RA, Bharani S, Prabhakar S. Frey's Syndrome Consequent to an Unusual Pattern of Temporomandibular Joint Dislocation: Case Report with Review of Its Incidence and Etiology. Craniomaxillofac Trauma Reconstr. 2013 Mar;6(1):1-8. doi: 10.1055/s-0032-1332210. [PubMed] [Free full text]
- Gordon AB, Fiddian RV. Frey's syndrome after parotid surgery. Am J Surg. 1976;132(1):54-8. [PubMed]
- Choi HG, Kwon SY, Won JY, Yoo SW, Lee MG, Kim SW, et al. Comparisons of three indicators for Frey's Syndrome: Subjective symptoms, Minor's starch iodine test, and infrared thermography. Clinical and experimental otorhinolaryngology. 2013;6(4):249-53.
- Scrivani SJ, Keith DA, Kulich R, Mehta N, Maciewicz RJ. Posttraumatic gustatory neuralgia: a clinical model of trigeminal neuropathic pain. J Orofac Pain. 1998;12(4):287-92. [PubMed]
- Loughner BA, Larkin LH, Mahan PE. Nerve entrapment in the lateral pterygoid muscle. Oral Surg Oral Med Oral Pathol. 1990;69(3):299-306. [PubMed]
- Chim H, Okada HC, Brown MS, Alleyne B, Liu MT, Zwiebel S, et al. The auriculotemporal nerve in etiology of migraine headaches: compression points and anatomical variations. doi: 10.1097/PRS.0b013e3182589dd5. [PubMed]
- Janis JE, Hatef DA, Thakar H, Reece EM, McCluskey PD, Schaub TA, et al. The zygomaticotemporal branch of the trigeminal nerve: Part II. Anatomic variations. Plast Reconstr Surg. 2010 Aug;126(2):435-42. doi: 10.1097/PRS.0b013e3181e094d7. [PubMed]
- Schmidt BL, Pogrel MA, Hakim-Faal Z. The course of the temporal branch of the facial nerve in the periorbital region. J Oral Maxillofac Surg. 2001;59(2):178-84. [PubMed]
- Anil A, Peker T, Turgut HB, Gulekon IN, Liman F. Variations in the anatomy of the inferior alveolar nerve. Br J Oral Maxillofac Surg. 2003;41(4):236-9. [PubMed]
- Kemp WJ 3rd, Tubbs RS, Cohen-Gadol AA. The innervation of the scalp: A comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int. 2011;2:178. doi: 10.4103/2152-7806.90699. [PubMed] [Free full text]
- Shankar H, Brethauer J. Ultrasound guided steroid injection for auriculo-temporal neuralgia. Anesthesiology. 2007;107:A903. doi: 10.1097/01.anes.0000290617.98058.d9. [Free full text]
- Sjaastad O, Saunte C, Hovdahl H, Breivik H, Gronbaek E. "Cervicogenic" headache. An hypothesis. Cephalalgia. 1983;3(4):249-56. [PubMed] [Free full text]
- Blume H, Atac M, Golnick J. Neurosurgical treatment of persistent occipital myalgia-neuralgia syndrome. In: Pfaffenrath V, Lundberg P-O, Sjaastad O, editors. Updating in Headache: Springer Berlin Heidelberg; 1985. p. 24-34.
- Beruto Y, Lentijo J, Ramus NM. Descodes de med y irugpract. Madrid. 1821;3:145-69.
- Dubuisson D. Treatment of occipital neuralgia by partial posterior rhizotomy at C1-3. J Neurosurg. 1995;82(4):581-6. [PubMed]
- Scattoni L, Di Stani F, Villani V, Dugoni D, Mostardini C, Reale C, et al. Great occipital nerve blockade for cluster headache in the emergency department: case report. J Headache Pain. 2006;7(2):98-100.[Free full text]
- Peres MF, Stiles MA, Siow HC, Rozen TD, Young WB, Silberstein SD. Greater occipital nerve blockade for cluster headache. Cephalalgia. 2002;22(7):520-2. [PubMed] [Free full text]
- Anthony M. Arrest of attacks of cluster headache by local steriod injections in the occipital myalgia-neuralgia syndrome. In: Pfaffenrath V, Lundberg PO, Sjaastad O, editors. Updating in Headache. Berlin: Springer-Verlag; 1985. p. 17-23.
- Cortijo E, Guerrero AL, Herrero S, Mulero P, Munoz I, Pedraza MI, et al. Hemicrania continua in a headache clinic: referral source and diagnostic delay in a series of 22 patients. J Headache Pain. 2012;13(7):567-9. [Free full text]
- Ashkenazi A, Matro R, Shaw JW, Abbas MA, Silberstein SD. Greater occipital nerve block using local anaesthetics alone or with triamcinolone for transformed migraine: a randomised comparative study. J Neurol Neurosurg Psychiatry. 2008;79(4):415-7. [PubMed] [Free full text]
- Gille O, Lavignolle B, Vital JM. Surgical treatment of greater occipital neuralgia by neurolysis of the greater occipital nerve and sectioning of the inferior oblique muscle. Spine (Phila Pa 1976). 2004;29(7):828-32. [PubMed]
- Shimizu S, Oka H, Osawa S, Fukushima Y, Utsuki S, Tanaka R, et al. Can proximity of the occipital artery to the greater occipital nerve act as a cause of idiopathic greater occipital neuralgia? An anatomical and histological evaluation of the artery-nerve relationship. Plast Reconstr Surg. 2007;119(7):2029-34; discussion 35-6. [PubMed]
- Natsis K, Baraliakos X, Appell HJ, Tsikaras P, Gigis I, Koebke J. The course of the greater occipital nerve in the suboccipital region: a proposal for setting landmarks for local anesthesia in patients with occipital neuralgia. Clin Anat. 2006;19(4):332-6. [PubMed]
- Becser N, Bovim G, Sjaastad O. Extracranial nerves in the posterior part of the head. Anatomic variations and their possible clinical significance. Spine (Phila Pa 1976). 1998 Jul 1;23(13):1435-41. [PubMed]
- Dash KS, Janis JE, Guyuron B. The lesser and third occipital nerves and migraine headaches. Plast Reconstr Surg. 2005;115(6):1752-8; discussion 9-60. [PubMed]
- Bovim G, Bonamico L, Fredriksen TA, Lindboe CF, Stolt-Nielsen A, Sjaastad O. Topographic variations in the peripheral course of the greater occipital nerve. Autopsy study with clinical correlations. Spine (Phila Pa 1976). 1991 Apr;16(4):475-8. [PubMed]
- Baer-Henney S, Tatagiba M, Samii M. Osteochondroma of the cervical spine causing occipital nerve neuralgia. Case report. Neurol Res. 2001;23(7):777-9. [PubMed]
- Kihara T, Shimohama S. Occipital neuralgia evoked by facial herpes zoster infection. Headache. 2006;46(10):1590-1. [PubMed]
- Star MJ, Curd JG, Thorne RP. Atlantoaxial lateral mass osteoarthritis. A frequently overlooked cause of severe occipitocervical pain. Spine (Phila Pa 1976). 1992;17(6 Suppl):S71-6. [PubMed]
- Ehni G, Benner B. Occipital neuralgia and the C1-2 arthrosis syndrome. J Neurosurg. 1984;61(5):961-5. [PubMed]
- Shim JH, Ko SY, Bang MR, Jeon WJ, Cho SY, Yeom JH, et al. Ultrasound-guided greater occipital nerve block for patients with occipital headache and short term follow up. Korean J Anesthesiol. 2011;61(1):50–4. doi: 10.4097/kjae.2011.61.1.50. [PubMed] [Free full text]
- Na SH, Kim TW, Oh SY, Kweon TD, Yoon KB, Yoon DM. Ultrasonic doppler flowmeter-guided occipital nerve block. Korean J Anesthesiol. 2010 Dec; 59(6):394-7. doi: 10.4097/kjae.2010.59.6.394. [PubMed] [Free full text]
- Palamar D, Uluduz D, Saip S, Erden G, Unalan H, Akarirmak U. Ultrasound-guided greater occipital nerve block: an efficient technique in chronic refractory migraine without aura? Pain Physician. 2015;18(2):153-62. [PubMed] [Free full text]
- Greher M, Moriggl B, Curatolo M, Kirchmair L, Eichenberger U. Sonographic visualization and ultrasound-guided blockade of the greater occipital nerve: a comparison of two selective techniques confirmed by anatomical dissection. Br J Anaesth. 2010 May;104(5):637-42. doi: 10.1093/bja/aeq052. [PubMed] [Free full text]