Azmat Riaz, MCPS, FCPS*, Rao Ali Shan Khan, FCPS**, Asjad Sharif, MCPS, FCPS*
*Assistant Professor; **Associate Professor
Department of Anesthesiology, Pain & Intensive Care, Combined Military Hospital, Rawalpindi, (Pakistan)
Correspondence: Dr Azmat Riaz, Consultant Anesthesiologist, Department of Anesthesiology, Pain & Intensive Care, New OT Complex, Combined Military Hospital, Rawalpindi-46000 (Pakistan); Cell: 0092-333-8163966; E-mail: azmatrt@yahoo.com
ABSTRACT
Objective: This comparative study was designed to assess the efficacy of zolmitriptan, a triptan widely employed as first-line therapy for migraine, in relieving post-dural puncture headache in parturients who were operated for cesarian section under spinal anesthesia.
Methodology: The study was carried out at department of Anesthesiology, Combined Military Hospital Rawalpindi, over a period of twelve months from August 2012 to July 2013. We enrolled 60 adult parturients who complained of post-dural puncture headache in post-partum period. They were divided into two equal groups of 30 each; Group-1 (Z-group) received zolmitriptan along with other supportive treatment for spinal headache and Group-2, control group (C-group) was given only supportive treatment. Efficacy of zolmitriptan in relieving post-dural puncture headache (PDPH) was studied and frequency of complications of the drug were noted.
Results: After 6 hours, 60% patients of Z-group got relief from headache. While in control group only 36.66% patients were pain free (p 0.016). After 12 hours, relief from headache was noted in 70% patients of zolmitriptan-group while in C-group only 46.66% were relieved (p 0.015). After a period of 24 hours results were 86.66% vs. 63.33% (p 0.006). After 48 hours, in Z-group 96.66% patients were pain free, while in C-group 63.33% were free from headache (p 0.001). There was no change in results for Z-group after 72 hours. In contrast, in C-group 80% were pain free and 20% patients were still symptomatic. Borderline statistical significance was present (p 0.046).
Conclusion: The study revealed that anti-migraine drug zolmitriptan in combination with supportive treatment is effective in relieving PDPH in parturients.
Keywords: Post-dural puncture headache; Spinal anesthesia; Zolmitriptan.
Citation: Riaz A, Khan RAS, Sharif A. Zolmitriptan is effective in relieving post-dural puncture headache in young parturients. Anaesth Pain & Intensive Care 2014;18(2):147-51
INTRODUCTION
In obstetric population unintentional dural puncture is one of the common major complications. The incidence of dural puncture in obstetrics practice in UK is 0.18-3.6%. Post-dural puncture headache (PDPH) is an important iatrogenic cause of patient morbidity in modern anesthesia.¹ The incidence of headache after spinal anesthesia varies greatly between studies.² The incidence of disabling headache is estimated to be between 0.3% – 45% following spinal anesthesia and up to 81% following accidental dural puncture during epidural insertion in the pregnant woman. The main factor determining the frequency of PDPH following spinal anesthesia is the size and design of the needle. It has been established beyond doubt that use of larger needles is associated with a greater incidence of PDPH. The incidence varies with the needle size and is ∼40% with a 22G needle; 3-25% with a 25G needle; 2–12% with a 26G Quincke needle and <2% with a 29G needle.3 Eighty percent of patients with dural puncture suffer from PDPH.4 By using progressively smaller needles and changing the design of needle tips from cutting Quincke needles to pencil-point needles, further reduced the incidence.5,6 Other risk factors for PDPH are multiple attempts during procedure, female sex, younger age group and obstetric population. The obstetric patient is at particular risk of headache because of sex, young age, and the widespread application of regional anesthesia.7 The accompanying symptoms are usually nausea, vomiting, neck stiffness, ocular complaints such as photophobia and diplopia, and auditory complaints like tinnitus.8
PDPH is usually a self-limiting process. If left untreated, 75% resolve within the first week and 88% resolve by 6 weeks, however early treatment is indicated if symptoms are troublesome for patient.9 Most treatments are geared towards lessening the pain and symptoms until the hole in the dura can heal, or at least until it can close to the point where the symptoms are tolerable. Once a diagnosis of PDPH is established, various treatment modalities are available, ranging from non-invasive pharmacological measures to invasive procedures. Conservative treatment includes bed rest, oral fluids, use of oral or IV caffeine and mild laxatives. Analgesics including paracetamol and /or NSAIDs are also prescribed to treat the headache. Some new pharmacological measures which have been recently tried include theophylline, ACTH hormone, desmopressin (DDAVP) and triptans.10
Triptans proved to be the most effective acute anti-migraine medications and are preferable for menstruation related migraine.11 Sumatriptan has been studied for its efficacy in treating PDPH with favorable results. It has been shown to be well tolerated and effective when administered in combination with analgesics.12 Relatively a new drug, zolmitriptan is a second generation triptan, and it is available in our country and being used as a first line drug for migraine. It has not yet been studied for PDPH. Our study is based on the assumption that zolmitriptan, like other triptans should show promising results for treatment of PDPH.14
METHODOLOGY
Approval was sought from hospital ethical committee for the study. We included young parturients in the study, who complained of moderate to severe post dural puncture headache on second or third postoperative day. Patients had history of spinal anesthesia with 24 or 25G Quincke needles, mostly performed by anesthesia residents. Patients with history of ischemic heart disease, PIH, chronic hypertension, cardiac, vascular, liver and renal impairment, or any other severe or disabling medical condition were not included in the study. Individuals with history of migraine, known hypersensitivity to study drugs, previous inadequate response to at least two triptans, currently using ergotamine or MAO-inhibitors were excluded as well. Patients were given the option of pharmacological treatment or invasive procedure (epidural blood patch). Patients who opted for pharmacological treatment were included in the study and divided into two groups of 30 each (Table 1). Ladies who were breast-feeding their neonates were given only supportive treatment, because the safety of study drug in lactating mothers has not yet been established. Patients were kept in the hospital for at least three days. Written informed consent was obtained from all patients prior to their inclusion in the study. Patients of both groups were given bed rest, Ringer’s solution 2 lit/day, along with oral fluids (including coffee and tea) and mefenamic acid 500 mg three times a day. Patients of zolmitriptan-group were given zolmitriptan (Zomig®) 5 mg stat and then 2.5 mg once daily for next two days. A 5-point visual analogue pain scale was used to describe the intensity of pain;
0 = no pain
1 = mild pain (pain which did not affect the everyday activity of patient)
2 = moderate pain (pain which was present on standing but relieved somewhat on lying down, confining them to bed)
3 = Severe pain (pain which didn’t even relieve on lying down)
4 = very severe pain (severe pain along with associated symptom i.e. nausea, tinnitus, neck stiffness etc.).
Patients were interviewed by a consultant in anesthesia regarding relief from headache after 6 hours and then at 12, 24, 48 and 72 hours. The end point of study was mild pain or no pain at all. Patients having complaints of nausea were given anti-emetic metoclopramide and those having neck stiffness were treated with oral tizanidine. Incidence of any adverse effects like tingling sensation, dizziness, tightness or heaviness in the chest, throat, neck, or jaws, drowsiness, and warmth sensation in the body were also noted.
RESULTS
Data are presented as Mean ± SD. Comparison between groups was performed by using Mann-Whitney U-test. The significance level was set at P ˂ 0.05. IBM SPSS Statistics 20 software was used for statistical analysis. Out of 30 patients in zolmitriptan-group 21 patients had had very severe headache (pain score 4) while 9 patients had severe headache (pain score 3). In control-group 23 patients were with very severe headache and rest of the 7 had severe headache. Six hours after the treatment 18 patients in zolmitriptan-group got relief from headache (mild pain-14, no pain-4), so 60% of our patients got relief. While in control group only 11 patients (36.66%) fell in the category of mild pain (no patient in no-pain category). This result was statistically significant (p-value 0.016). After 12 hours, in zolmitriptan-group 3 more patients (70%) were relieved from headache (mild pain-16, no pain-5). In control group 14 patients (46.66%) were relieved from headache (mild pain-13, no pain-1). This again showed a statistical significance (p-value 0.015).
Table 1: Patient characteristics and duration of surgery.
|
Patient Characteristics |
Zolmitriptan Group (n=30) |
Control Group (n=30) |
| Age (Years) |
28.5 ± 3.71 |
26.93 ± 4.17 |
| ASA-Status I/II |
17/13 |
19/11 |
| Weight (Kg) mean ± SD |
56.7 ± 6.22 |
63.2 ± 7.51 |
| Duration of Surgery (Min) mean ± SD |
62 ± 9.70 |
60.83 ± 10.17 |
Table 2. Pain scores of both groups. Values are in number (n) and percentages (%).
|
VAS score |
Zolmitriptan group (n=30) |
Control group (n=30) |
|
00 Hours |
||
| 4= Very Severe |
21 (70) |
23 (76.66) |
| 3= Severe |
9 (30) |
7 (23.33) |
| 2= Moderate |
0 |
0 |
| 1= Mild |
0 |
0 |
| 0= No Pain |
0 |
0 |
|
06 Hours |
||
| 4= Very Severe |
1 (3.33) |
3 (10) |
| 3= Severe |
3 (10) |
8 (26.66) |
| 2= Moderate |
8 (26.66) |
8 (26.66) |
| 1= Mild |
14 (46.66) |
11 (36.66) |
| 0= No Pain |
4 (13.33) |
0 |
|
12 Hours |
||
| 4= Very Severe |
0 |
0 |
| 3= Severe |
2 (6.66) |
9 (30) |
| 2= Moderate |
7 (23.33) |
7 (23.33) |
| 1= Mild |
16 (53.33) |
13 (43.33) |
| 0= No Pain |
5 (16.66) |
1 (3.33) |
|
24 Hours |
||
| 4= Very Severe |
0 |
0 |
| 3= Severe |
1 (3.33) |
3 (10) |
| 2= Moderate |
3 (10) |
11 (36.66) |
| 1= Mild |
0 |
0 |
| 0= No Pain |
26 (86.66) |
16 (53.33) |
|
48 Hours |
||
| 4= Very Severe |
0 |
0 |
| 3= Severe |
0 |
1 (3.33) |
| 2= Moderate |
1 (3.33) |
10 (33.33) |
| 1= Mild |
0 |
0 |
| 0= No Pain |
29 (96.66) |
19 (63.33) |
|
72 Hours |
||
| 4= Very Severe |
0 |
0 |
| 3= Severe |
0 |
0 |
| 2= Moderate |
1 (3.33) |
6 (20) |
| 1= Mild |
0 |
0 |
| 0= No Pain |
29 (96.66) |
24 (80) |
After a period of 24 hours in zolmitriptan-group only 4 patients were left with significant symptoms, while 26 patients (86.66%) were now pain free. In control group 11 patients were still symptomatic and rest of the 19 patients (63.33%) were now pain free. Again our results were statistically significant (p-value 0.006).
After a period of 48 hours, in zolmitriptan-group only one patient was left with symptoms, and that too moderate pain (pain score-3), while our 29 patients (96.66%) were now pain free (Table 2). While in control-group 11 patients were still symptomatic and 19 patients (63.33%) were free from headache. The statistical significance was again present (p-value 0.001).
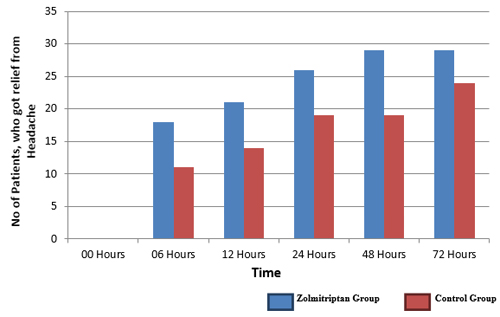
After 72 hours for zolmitriptan-group results were same, with one patient still complaining of headache and 29 were pain free. In contrast, in control-group 6 patients were still symptomatic (but all with moderate pain, no one in severe category) and 24 patients were now relieved (80%). The statistical significance was borderline (p-value 0.046) (Figure 1).
Figure 1: Comparison of complete pain relief in both groups.
Regarding side effects of the drug, only three patients from zolmitriptan-group complained of temporary feeling of fatigue.
DISCUSSION
This is the first study involving the use of anti-migraine drug zolmitriptan (Zomig® AstraZeneca) as acute treatment of PDPH. The International Headache Society describes PDPH as ‘headache that worsens within 15 min after sitting or standing and improves within 15 min after lying, with at least one of the following; neck stiffness, tinnitus, photophobia or nausea. There should be a history of dural puncture and the headache should develop within five days after dural puncture’. Although PDPH is a self-limiting and nonfatal condition, its postural nature prevents the patient from performing routine activity and many make them anxious, resentful or depressed. It can also deter patients from accepting the same anesthetic in future. PDPH classically is occipitofrontal and often radiates to the neck and shoulders. Pain is exacerbated by sitting and standing, and alleviated by lying flat.15 Other associated symptoms include nausea, vomiting, hearing loss, tinnitus, vertigo, dizziness, diplopia and paresthesia of the scalp. The obstetric patient is usually aware that her headache is an iatrogenic problem. Headache may make it difficult to care for the newborn and to interact with other family members. It is, therefore, important to give the parturient a thorough explanation of the reason for the headache, the anticipated time course and the therapeutic options available.
Conventional treatment for PDPH includes bed rest, specific postures and additional fluids above those required for maintenance. Analgesics including paracetamol and NSAIDs, in conjunction with antiemetic, may control the symptoms and so reduce the need for interventional therapy. Caffeine is a CNS stimulant which also has a cerebral vasoconstrictive effect. Single doses of intravenous as well as oral caffeine (250 mg and 300 mg respectively) have been shown to relieve mild PDPH but the effect is transient.16 Caffeine occasionally is associated with post-partum seizures.17 Cosyntropin or synthetic ACTH, and hydrocortisone are believed to work by stimulating the adrenal gland and increasing CSF secretion.18 These drugs and theophylline have been found to be effective in treating PDPH after failed epidural blood patch.19
The 5-hydroxytryptamine (5HT 1B/1D) agonists, collectively known as triptans, are a major advance in the treatment of migraine.20 The beneficial effects of the triptans in patients with migraine are related to multiple mechanisms of action at sites implicated in the pathophysiology of migraine. Sumatriptan has been shown to be well tolerated and effective in providing pain relief when administered in the mild pain phase, also in combination with analgesics.21 Carp H et al tested efficacy of sumatriptan in six cases of PDPH and found it very effective in relieving headache. Five out of six cases were relieved from headache completely.22 Lhuissier C et al also found sumatriptan beneficial in relieving PDPH. 23 Second generation triptans such as zolmitriptan, naratriptan, rizatriptan and more recently almotriptan and frovatriptan have been also successfully tested. 24 Zolmitriptan is a 5-HT 1B and 5-HT 1D receptor agonist with cerebral vasoconstrictive effects. It is used primarily in the treatment of migraine. It promotes cerebral vasoconstriction in a similar way to caffeine.
In our study 18 out of 30 patients (60%) got relief from headache within six hours of starting treatment with zolmitriptan. This finding was consistent with Gallagher RM, et al, who found in their study that zolmitriptan, 2.5 mg and 5 mg, was at least as effective as sumatriptan, 25 mg or 50 mg, for all parameters studied. Zolmitriptan 2.5 mg, was significantly more effective than sumatriptan 50 mg in terms of headache response at 4 hours.25 Patients taking zolmitriptan were significantly more likely to have pain relief over 24 hours than those taking sumatriptan. Zolmitriptan is effective in the treatment of migraine associated with menstruation and migraine with aura. There is no tachyphylaxis following repeated doses for multiple attacks of migraine over a prolonged period of time. Compared to placebo, the incidence of persistent migraine headache is reduced by zolmitriptan and recurrent migraine headache occurs less frequently. Zolmitriptan has also shown efficacy in the treatment of persistent and/or recurrent migraine headache. Comparative clinical studies done by Dowson AJ and Charlesworth B have shown that zolmitriptan has similar or superior efficacy to sumatriptan in the treatment of migraine.26 It inhibits the peripheral trigemino-vascular system and is able to access central sites in the brainstem involved in processing cranial pain.
One interesting result of our study is the borderline statistical significance (p-value 0.046) after 72 hours as the relief was 96.66% vs. 80% in zolmitriptan-group. This finding is consistent with Goldszmidt E, et al9 who found that most of the times PDPH is a self-limiting process and if left untreated, 75% resolve within the first week and 88% resolve by 6 weeks. However, they recommend that early treatment should be started if the symptoms are troublesome for the patient. As with other 5-HT1 agonists, sensations of tightness, pain, and pressure in the chest, throat, neck, and jaw can occur after treatment with zolmitriptan and is usually non-cardiac in origin.
It is not known whether zolmitriptan is excreted in human milk. Animal studies have reported milk levels equivalent to maternal plasma levels at one hour after dose administration, and four times higher than plasma levels at four hours after dose administration (in the milk of lactating rats).
This was the first ever study on zolmitriptan as a first line drug for PDPH. Lack of availability of published data and references definitely limit our study. Our study showed promising results.
CONCLUSION
There is still no specific non-invasive treatment available for PDPH. We found in our small scale study that anti-migraine drug zolmitriptan is effective in relieving PDPH and can be employed for this purpose with satisfactory results. We suggest that controlled large scale studies be undertaken on zolmitriptan and other newer triptans for their beneficial effect on PDPH.
Financial Support: Departmental sources only
Disclaimers: None
Conflict of Interest: None
REFERENCES
- Tumbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth 2003;91:718-729. [PubMed][Free Full Text]
- Hayes NE, Wheelahan JM, Ross A. Self-reported post-discharge symptoms following obstetric neuraxial blockade. Int J Obstet Anesth 2010;19:405-9. [PubMed]
- Santanen U. Comparison of 27-gauge (0.41-mm) Whitacre and Quincke spinal needles with respect to post-dural puncture headache and non-dural puncture headache. Acta Anaesthesiol Scand 2004;48:474-9. [PubMed]
- Stella CL, Jodicke CD, How HY, Harkness UF, Sibai BM. Postpartum headache: Is your work-up complete? Am J Obstet Gynecol 2007;196:318.e1-7. [PubMed]
- Weir EC. The sharp end of the dural puncture. Br Med J 2000;320:127-8. [PubMed][Free Full Text]
- Klein AM, Loder E. Postpartum headache. Int J Obstet Anesth 2010;19:422-30. [PubMed]
- Goldszmidt E, Kern R, Chaput A, Macarthur A. The incidence and etiology of postpartum headaches: A prospective cohort study. Can J Anesth 2005;52:971-7. [PubMed]
- Nafiu OO, Salam RA, Elegbe EO. Post dural puncture headache in obstetric patients: Experience from a West African teaching hospital. Int J Obstet Anesth 2007;16:4-7. [PubMed]
- Connely NR, Parker RK, Rahimi A, Gibson CS. Sumatriptan in patients with postdural puncture headache. Headache 2000;40:316-9. [PubMed]
- Solomon GD, Cady RK, Klapper JK, Earl NL. Clinical efficacy and tolerability of 2.5 mg zolmitriptan for the acute treatment of migraine. Neurology 1997;49:1219-25. [PubMed]
- Choi PT, Galinski SE, Lucas S, et al. Examining the evidence in anesthesia literature: a survey and evaluation of obstetrical postdural puncture headache reports. Can J Anaesth 2002;49:49-56. [PubMed]
- Sprigge JS, Harper SJ. Accidental dural puncture and post dural puncture headache in obstetric anaesthesia: Presentation and management: A 23-year survey in a district general hospital. Anaesthesia 2008;63:36-43. [PubMed]
- Van de Velde M, Schepers R, Berends N, Vandermeersch E, De Buck F. Ten years of experience with accidental dural puncture and post-dural puncture headache in a tertiary obstetric anaesthesia department. Int J Obstet Anesth 2008;17:329-35. [PubMed]
- Benzon HT, Wong CA: postdural puncture headache: mechanisms, treatment, and prevention (editorial). Reg Anesth Pain Med 2001;26:293-5. [PubMed]
- Kuczkowski KM. The management of accidental dural puncture. Anaesthesia 2006;61:68. [PubMed]
- Halker RB. Caffeine for the prevention and treatment of postdural puncture headache: Debunking the myth. Neurologist 2007;13:323-7. [PubMed]
- Camann WR, Murray RS, Lambert DH. Effects of oral caffeine on postdural puncture headache. A double-blind, placebo-controlled trial. Anesth Analg 1990;70:181-4. [PubMed]
- Atulkumar MK, Fostor PA. Adrenocorticotropic hormone infusion as a novel treatment for postdural puncture headache. Reg Anesth 1997;22:432-434. [PubMed]
- Ergun U, Say B, Ozer G et al. Intravenous theophylline decreases post-dural puncture headaches. J Clin Neurosci. 2008;15:1102-4. [PubMed]
- Pascual J, Munoz R, Leira R. An open preference study with sumatriptan 50 mg and zolmitriptan 2.5 mg in 100 migraine patients. Cephalalgia 2001;21:680–4. [PubMed][Free Full Text]
- Hodgson C, Roitberg-Henry A. The use of sumatriptan in the treatment of postdural puncture headache. Anaesthesia 1997;52:808. [PubMed]
- Carp H, Singh PJ, Vadhera R, Jayaram A. Effects of the Serotonin-Receptor Agonist Sumatriptan on Post-dural Puncture Headache: Report of Six Cases. AnesthAnalg 1994; 79:180-2. [PubMed]
- Lhuissier C, Mercier FJ, Doucas M, Benhamou D. Sumatriptan: an alternative to epidural blood patch? Anaesthesia 1996;54:1078. [PubMed]
- Bussone G. Frovatriptan for the prevention of postdural puncture headache. Cephalalgia 2007;27:809-13. [PubMed]
- Gallagher RM, Dennish G, Spierings EL, Chitra R. A comparative trial of zolmitriptan and sumatriptan for the acute oral treatment of migraine. Headache 2000;40:119-28. [PubMed]
- Dowson AJ. Charlesworth B. Review of zolmitriptan and its clinical applications in migraine. Expert Opin Pharmacother 2002;3:993-1005. [PubMed]