Tomoki Nishiyama, MD, PhD
Department of Anesthesiology, Shinagawa Shishokai Hospital, 1-29-7, Kita-Shinagawa, Shinagawa-ku, Tokyo, 140-0001 (Japan)
Correspondence: Tomoki Nishiyama, MD, PhD, Department of Anesthesiology, Shinagawa Shishokai Hospital, 1-29-7, Kita-Shinagawa, Shinagawa-ku, Tokyo, 140-0001 (Japan); Tel: +81-3-5781-0700; Fax: +81-3-5781-0828; E-mail: nishit-tky@umin.ac.jp
ABSTRACT
Objective:Arterial oxygen pressure (PaO2) may decrease at induced hypotension during general anesthesia by nicardipine or prostaglandin E1 (PG). The present study was performed to compare the effects of nicardipine and PG on PaO2 during induced hypotension in general anesthesia.
Methodology: This randomized controlled non-blind study was conducted at our operating room at the University Hospital.
Fifty patients aged 40 to 65 years for resection of brain tumor were enrolled in the study. During general anesthesia with isoflurane, nitrous oxide and fentanyl, when hemodynamics were stable, PG 0.05 µg/kg/min, or 0.1 µg/kg/min, or nicardipine 0.5 µg/kg/min, or 1.0 µg/kg/min was administered for two hours in 10 patients each. Another 10 patients were reserved as the control.
Arterial blood pressure, heart rate, percutaneous oxygen saturation (SpO2), end-tidal carbon dioxide pressure (EtCO2), and arterial oxygen (PaO2) and carbon dioxide (PaCO2) pressures were measured until 90 min after stop of administration of PG or nicardipine. Ratio of PaO2 and oxygen fraction (P/F ratio) was calculated.
Results: Both PG and nicardipine decreased blood pressure similarly with increase in heart rate. P/F ratio decreased only with PG.
Conclusion. The use of prostaglandin E1 to induce hypotension during general anesthesia is associated with a decreased PaO2, while nicardipine has no effect. This difference in effect on PaO2 is important in selecting an agent to induce hypotension in neurosurgery.
Keywords: Hypotension; Nicardipine; Prostaglandin E1; Arterial oxygen pressure
Citation: Nishiyama T. Randomized controlled trial to compare the effects of nicardipine and prostaglandin E1 induced hypotension on arterial oxygen pressure in patients undergoing brain tumor resection under general anesthesia. Anaesth Pain & Intensive Care 2015;19(1):3-7
INTRODUCTION
To induce hypotension for decreasing blood loss during general anesthesia in neurosurgery, nicardipine or prostaglandin E1 (PG) is often infused. It is usually considered that systemic vasodilation induces pulmonary vascular dilation and increases pulmonary blood flow, which increases intrapulmonary shunt.1 Therefore, arterial oxygen pressure (PaO2) may decrease, which is sometimes critical in neurosurgery because brain is easily damaged by short lasting hypoxia.
In animal study, PG inhibits hypoxic pulmonary vasoconstriction, which may worsen pulmonary gas exchange.2 In dogs, nicardipine decreased systemic and pulmonary vascular resistance, and increased cardiac output, intrapulmonary shunt, which decreased PaO2.3 In human study, PG decreased PaO2 significantly in the adult respiratory distress syndrome.4 We could not find any human studies about the effects of nicardipine on PaO2. Therefore, the present study was performed to compare the effects of nicardipine and PG induced hypotension on PaO2 during general anesthesia for brain tumor resection.
METHODOLOGY
This is a non-blind randomized controlled study. After the approval of the ethics committee of the hospital under the standards of the Helsinki Declaration and informed consent from patients, 50 patients aged 40 to 65 years for resection of brain tumor were enrolled in this study. They were divided into five groups at random by an envelope method. Those who had respiratory, cardiac, renal, or liver diseases, and who had taken prostaglandin, or calcium antagonists before surgery were excluded.
Without any premedication, anesthesia was induced with midazolam 0.1 mg/kg, fentanyl 4 µg/kg, and endotracheal intubation was facilitated with vecuronium 0.15 mg/kg. Anesthesia was maintained with oxygen 2 L/min, nitrous oxide 4 L/min, isoflurane 0.5 to 1.0 %, and fentanyl 6 to 10 µg/kg (including induction dose).Radial artery was cannulated to measure blood pressure and arterial blood gas. Lactated Ringers solution was infused at 4 – 5 ml/kg/h and to keep urine volume more than 1 ml/kg/h. Twenty % mannitol 300 ml was infused just after craniotomy. Ventilation was adapted to keep end-tidal carbon dioxide tension (EtCO2)between 30 and 35 mmHg.
After microsurgical procedure started and when blood pressure and heart rate were stable as ±5% variation for 30 min, administration of PG 0.05 µg/kg/min (Group PG0.05), PG 0.1 µg/kg/min (Group PG0.1), nicardipine 0.5 µg/kg/min (Group N0.5), or nicardipine 1.0 µg/kg/min (Group N1) started and stopped at two hours later in 10 patients each. Another 10 patients were served as the control without infusion of any vasodilators.
Arterial blood pressure, heart rate, percutaneous oxygen saturation (SpO2), EtCO2, PaO2, and PaCO2 were measured every 5 min until 90 min after stop of administration of PG or nicardipine. PaO2 / FIO2 (fraction of inspiratory oxygen) was calculated as P/F ratio.
Data were shown as mean ± standard deviation or number of patients.Power analysis was performed to detect the intra- and inter- group differences of measured parameters with power of 0.95 and effect size of 0.25 using G PowerTM software.5 Statistical analysis was performed with the chi-square test and factorial analysis of variance (ANOVA) for demographic data, and repeated ANOVA for measured parameters followed by Student-Neuman-Keuls test as a post hoc analysis. A p value less than 0.05 was considered to be statistically significant.
RESULTS
By the power analysis, 50 patients had enough power to detect the difference. Demographic data were not different among the five groups (Table 1). Isoflurane usage was not different between the two groups (Table 2).
Table 1: Demographic data of the patients
|
Parameter |
Control |
PG0.05 |
PG0.1 |
N0.5 |
N1 |
| Age (years) |
53 ± 8 |
58 ± 7 |
52 ± 5 |
55 ± 8 |
54 ± 6 |
| Gender (Male/Female) |
5/5 |
7/3 |
4/6 |
6/4 |
4/6 |
| Body weight (kg) |
59 ± 7 |
62 ± 8 |
60 ± 9 |
57 ± 6 |
59 ± 6 |
| Height (cm) |
162 ± 6 |
158 ± 8 |
160 ± 6 |
159 ± 7 |
164 ± 7 |
| Duration of surgery (min) |
485 ± 74 |
427 ± 63 |
466 ± 69 |
493 ± 80 |
448 ± 78 |
Legend: PG0.05, prostaglandin E1 0.05 µg/kg/min; PG0.1, prostaglandin E1 0.1 µg/kg/min; N0.5, nicardipine 0.5 µg/kg/min; N1, nicardipine 1 µg/kg/min
Data shown as mean ± standard deviation
Table 2: Data showing isoflurane use
|
Parameter |
Control |
PG0.05 |
PG0.1 |
N0.5 |
N1 |
| Isoflurane consumption (MAC.h) |
7.5 ± 2.0 |
6.8 ± 1.8 |
7.1 ± 2.2 |
7.7 ± 2.3 |
6.7 ± 2.1 |
| Maximum isoflurane concentration (MAC) |
1.4 ± 0.3 |
1.2 ± 0.4 |
1.1 ± 0.3 |
1.2 ± 0.3 |
1.3 ± 0.2 |
| Minimum isoflurane concentration (MAC) |
0.6 ± 0.1 |
0.5 ± 0.2 |
0.6 ± 0.2 |
0.7 ± 0.2 |
0.6 ± 0.3 |
Legend: PG0.05, prostaglandin E1 0.05 µg/kg/min; PG0.1, prostaglandin E1 0.1 µg/kg/min; N0.5, nicardipine 0.5 µg/kg/min; N1, nicardipine 1 µg/kg/min; MAC, minimum alveolar concentration
Data shown as mean ± standard deviation
PG decreased blood pressure dose dependently and returned to the control level in 30 min after stop of administration in Group PG0.05 and in 45 min in Group PG0.1 (Figure 1). Both Group N0.5 and Group N1 had the same level of blood pressure decrease, and blood pressure returned to the control level in 15 min after stop of administration in both Groups N0.5 and N1.
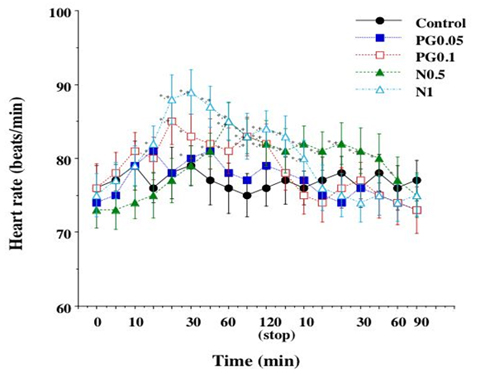
Heart rate increased in all groups (Figure 2). Group N1 had its peak increase at 30 min and returned to the control level in 10 min after stop of administration. Group N0.5 had its peak increase at 60 min and returned in 60 min after stop of administration. PG0.1 group had its peak increase at 20 min and returned in 60 min after start of administration. PG0.05 group had its peak increase at 15 min and returned in 45 min after start of administration.
Figure 1: Comparison of blood pressure in the groups
Mean ± standard deviation; PG0.05, prostaglandin E1 0.05 µg/kg/min; PG0.1, prostaglandin E1 0.1 µg/kg/min; N0.5, nicardipine 0.5 µg/kg/min; N1, nicardipine 1 µg/kg/min; *, P < 0.05 vs. the value at time 0; +, P < 0.05 vs. the value of the Control group
Figure 2: Comparison of heart rate in the groups
Mean ± standard deviation; PG0.05, prostaglandin E1 0.05 µg/kg/min; PG0.1, prostaglandin E1 0.1 µg/kg/min; N0.5, nicardipine 0.5 µg/kg/min; N1, nicardipine 1 µg/kg/min; *, P < 0.05 vs. the value at time 0; +, P < 0.05 vs. the value of the Control group
SpO2 was ≧98% in every patient and did not have any differences among the groups. EtCO2 was in the range between 30 and 35 mmHg during the study in all patients. P/F ratio decreased only in Groups PG0.05 and PG0.1 with its peak decrease at around 45 min after start of administration, and returned to the control levels in 5 min after stop of administration (Figure 3).
Figure 3: Comparison of P/F ratio in the groups

PaO2/FIO2; Mean ± standard deviation; PaO2, arterial oxygen pressure; FIO2, fraction of inspiratory oxygen; PG0.05, prostaglandin E1 0.05 µg/kg/min; PG0.1, prostaglandin E1 0.1 µg/kg/min; N0.5, nicardipine 0.5 µg/kg/min; N1, nicardipine 1 µg/kg/min; *, P < 0.05 vs. the value at time 0; +, P < 0.05 vs. the value of the Control group
DISCUSSION
There are some animal experiments of the effects of PG on PaO2. In dogs, PG inhibited hypoxic pulmonary vasoconstriction induced by vasodilation at 5 µg/kg/min but not at 1.25 µg/kg/min.6 PG 0.2 to 0.4 µg/kg/min decreased pulmonary vascular resistance and shunt, and increased PaO2 in dogs.7 Oxygen delivery remained unchanged or increased since the cardiac index increased with PG 0.025 to 0.1 µg/kg/min during one-lung ventilation in pigs.8 From these animal experiments, the doses of PG used in the present study might increase PaO2 by decreasing shunt and increasing cardiac output. However, our present clinical results showed the decrease in PaO2 (P/F ratio) with PG 0.05 µg/kg/min and 0.1 µg/kg/min.
In human studies, PG induced systemic hypotension and increased intrapulmonary shunt after heart transplantation.9 Naeije et al.10 showed that PG 0.02 to 0.04 µg/kg/min is a potent pulmonary vasodilator with minimal effects on gas exchange in pulmonary hypertension secondary to chronic obstructive pulmonary disease. PG 0.007 to 0.135 µg/kg/min decreased pulmonary arterial pressure, pulmonary vascular resistance, and pulmonary capillary wedge pressure, but did not change intrapulmonary shunt during surgery in patients with pulmonary hypertension.11 However, Melot et al. showed that PG 0.02 to 0.04 µg/kg/min increased shunt with no significant change in the pattern of ventilation-perfusion ratio (VA/Q) distribution, decreased PaO2 significantly in the adult respiratory distress syndrome.4 These studies have been done in patients with pulmonary hypertension and showed different results provably due to different severity of pulmonary hypertension. However, our study was performed in patients without pulmonary hypertension, and PaO2 decreased, maybe by increased shunt.
In dogs, nicardipine decreased systemic and pulmonary vascular resistance, and increased cardiac output, intrapulmonary shunt, which decreased PaO2.3 In addition, nicardipine decreases PaO2 due to ventilation-perfusion mismatch by inhibition of hypoxic pulmonary vasoconstriction induced by vasodilation. However, nicardipine increases cardiac output, which increases oxygen delivery.12 In human study,13 nicardipine does not dilate pulmonary vessels, and does not increase pulmonary shun. Therefore, PaO2 did not decrease with nicardipine in the present study.
CONCLUSION
In conclusion,to induce hypotension in general anesthesia for neurosurgery, the use of prostaglandin E1 is associated with a decreased PaO2, while nicardipine has no effect. This difference in effect on PaO2 is important in selecting an agent to induce hypotension in neurosurgery.
Source of funding: Nil.
Conflict of interest: None declared.
REFERENCES
- Fisher J, Borer JS, Moses JW, Goldberg HL, Niarchos AP, Whitman HH 3rd,et al. Hemodynamic effects of nifedipine versus hydralazine in primary pulmonary hypertension. Am J Cardiol 1984;54(6):646-650.[PubMed]
- Ishibe Y, Shiokawa Y, Umeda T, Uno H, Nakamura M, Izumi T. Prostaglandin E1 antagonizes hypoxic pulmonary vasoconstriction but reduces systemic blood pressure in dogs. Crit Care Med 1998;26(1):126-131.[PubMed]
- Casthely PA, Villanueva R, Rabinowitz, L, Gandhi P, Litwak B, Fyman PN. Intrapulmonary shunting during deliberate hypotension with nifedipine, diltiazem and labetalol in dogs. Can Anaesth Soc J 1985;32(2):119-23. [PubMed]
- Melot C, Lejeune P, Leeman M, Moraine JJ, Naeije R. Prostaglandin E1 in the adult respiratory distress syndrome. Benefit for pulmonary hypertension and cost for pulmonary gas exchange. Am Rev Respir Dis 1989;139(1):106-10. [PubMed]
- Faul F, Erdfelder E, Lang AG, Buchner A. G Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39(2):175-91. [PubMed]
- Weir EK, Reeves JT, Grover RF. Prostaglandin E1 inhibits the pulmonary vascular pressor response to hypoxia and prostaglandin F2 alpha. Prostaglandins 1975;10(4):623-31. [PubMed]
- Chen TL, Ueng TH, Huang CH, Chen CL, Huang FY, Lin CJ. Improvement of arterial oxygenation by selective infusion of prostaglandin E1 to ventilated lung during one-lung ventilation. Acta Anaesthesiol Scand 1996;40(1):7-13. [PubMed]
- Bund M, Henzler D, Walz R, Rossaint R, Piepenbrock S. Cardiopulmonary effects of intravenous prostaglandin E1 during experimental one-lung ventilation. Thorac Cardiovasc Surg 2006: 54(5):341-47. [PubMed]
- Kieler-Jensen N, Lundin S, Ricksten SE. Vasodilator therapy after heart transplantation: effects of inhaled nitric oxide and intravenous prostacyclin, prostaglandin E1 and sodium nitroprusside. J Heart Lung Transplant 1995;14(3):436-43. [PubMed]
- Naeije R, Melot C, Mols P, Hallemans R. Reduction in pulmonary hypertension by prostaglandin E1, in decompensated chronic obstructive pulmonary disease. Am Rev Respir Dis 1982;125(1):1-5. [PubMed]
- Heerdt PM, Weiss CI. Prostaglandin E1 and intrapulmonary shunt in cardiac surgical patients with pulmonary hypertension. Ann Thorac Surg 1990;49(3):463-5. [PubMed]
- Mookherjee S, Keighley JF, Warner RA, Bowser MA, Obeid AI. Hemodynamic, ventilatory and blood gas changes during infusion of sodium nitroferricyanide (Nitroprusside): Studies in patients with congestive heart failure. Chest 1977;72(3):273-8. [PubMed] [Free full text]
- Watoh Y, Tanaka A. Right ventricular performance during hypotension induced by prostaglandin E1, nicardipine HCL, glycerine trinitrate, and isosorbide dinitrate. J Anesth 1997;11(2):105-10. [PubMed]