Need to close the ‘closed suction in-line catheter’ port!
Manpreet Singh, MD, FCCP, FIMSA, Dheeraj Kapoor, MD, FCCP
Department of Anesthesiology and Intensive Care, Government Medical College and Hospital, Sector 32, Chandigarh, India-160030
Correspondence: Dr Manpreet Singh, 1223 A, GMCH Campus, Sec 32 B, Chandigarh, (India); Ph:+919646121503; E-mail:manpreetdawar@hotmail.com
Infection sources in ICU require utmost vigilance and care. We hereby describe another probable source of infection in patients of ICU where closed suction systems (CSS) are applied for uninterrupted ventilation even during suctioning. Growth of microorganisms increases, whenever these organisms get optimum environment for their multiplication. Closed suction inline catheter systems (CISC) are most often used in patients who are critical and require ventilation for prolonged period. Numerous CSS are marketed by manufacturers with one advantage or other. Kollef et al suggested that there is colonization of microorganisms in lower respiratory tract when such systems are placed over 24 hours1. Recently, numerous studies suggested that the use of CISC for more than 24 hours did not increase the risk of ventilator –associated pneumonia and it might be safe for patients2. Some manufacturers claim that this device can safely be attached for 72 hours continuously with low infection risk.
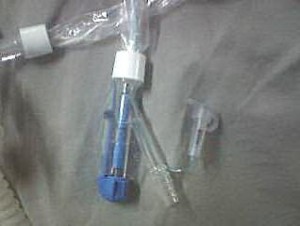
We routinely use these suction systems in patients on high PEEP ventilation. The manufacturer3 claims some advantages in one CSS with many attachments. This dual lumen CISC (Portex ®Suction ProTM 72) contains various ports .These ports are:
Port 1: Attached to endotracheal tube connecter
Port 2: Suctioning port that opens manually (Figure 1)
Port 3: For connection in-line with ventilator inspiratory circuit
Port 4: For attachment of supplied connectors (for nebulisation etc.)
This novel device produces great flow, high value and according to manufacturer it can be used for 72 hours. It provides an unobstructed evacuation pathway that helps make it easier to remove secretions, without the risk of cross contamination and the lockable end cap prevents inadvertent suctioning3.
Figure 1: The site (white arrow) where capping is required
It aids in disconnection of the catheter from the patient’s endotracheal or tracheostomy tubes and a patient label with day-of-the-week stickers are provided to monitor length of use. It is also a time saving solution for respiratory therapists, nurses and medical facilities.
After having used it in various patients in our ICU, it was observed that the suction port provided for attachment of the suction catheter, must have some capping or must have been manufactured in a way so that it does not act as a source of infection in critically ill and immunocompromised patients. It remains uncapped for 72 hours, and it may come into contact with some unsterile things. This might become a continuous source of infection and a place for microorganisms growth. The literature is abundant with reports of infections with respiratory tract secretions or catheter tips as a source, but not from these unusual sites. Further studies are required to samples these sites of CSS devices; the manufacturers may be suggested to take care of these sites either by capping or some coverings that can be removed while suctioning and reapplied afterwards.
References
1.Koller MH, Prentice D, Shapiro SD, Fraser VJ,Silver P, Trovilllion E, Wellitz P, von Harz B, St John R: Mechanical ventilation with or without daily changes of in-line suction catheters. Am Resp Crit Care med 1997; 156:466-472.
2. Inglis TJ, Lim TM,Ng ML,Tang EK,Hui KP. Structural features of tracheal tube biofilm formed during prolonged mechanical ventilation .Chest 1995;108:1049-1052.
3. Manufacturer’s manual- Smiths Medical International Limited.,UK. www.smiths-medical.com.