Abdullah Özer1, Faruk Metin Çomu2, Hüseyin Demirtaş3, Yiğit Kılıç1, Barış Mardin1, Levent Öztürk4, Erkan İriz1, Mustafa Arslan5, Ayşegül Küçük6
1Department of Cardiovascular Surgery, Gazi University Medical Faculty, Ankara-Turkey.
2Department of Physiology, Kırıkkale University Medical Faculty, Kirikkale- Turkey.
3Department of Cardiovascular Surgery, Yüksek Ihtisas Hospital, Kirikkale-Turkey.
4Department of Anaesthesiology and Reanimation, Yıldırım Beyazit University Medical Faculty, Ankara-Turkey.
5Department of Anaesthesiology and Reanimation, Gazi University Medical Faculty, Ankara-Turkey.
6Department of Physiology, Dumlupinar University Medical Faculty, Kütahya- Turkey
Correspondence: Dr. Mustafa Arslan, Gazi University Medical Faculty, Department of Anesthesiology and Reanimation, 06510 Ankara-Türkiye; Tel: 90 312 202 67 39; (GSM) 90 533 422 85 77; E-mail: mustarslan@gmail.com
ABSTRACT
Background & Objective: Acute ischemia reperfusion (IR) injury observed in the lower extremities occurs especially when a temporary cross-clamp is applied to the abdominal aorta during aortic surgery. Preoperative pregabalin has been used as a part of multimodal analgesia in postoperative pain treatment in recent years. Pregabalin has become one of the increasingly common agents in postoperative analgesia. In this study, we aimed to investigate the effect of pregabalin on erythrocyte deformability in rats undergoing IR.
Methodology: 24 male Wistar albino rats weighing between 200-250 g were used in the study. Rats were randomly divided into 4 groups of 6 rats each (Control, Ischemia-Reperfusion (IR), IR-Pregabalin 50 mg (50 mg/kg), IR-Pregabalin 200 mg (200 mg/kg). Pregabalin was administered intraperitoneally 30 min before the procedure. An atraumatic microvascular clamp was placed across the infrarenal abdominal aorta in the IR groups. Following 120 min of ischemia, the clamp was removed and reperfusion was continued for 120 min. All rats were euthanized by intraperitoneal administration of ketamine (100 mg/kg) and taking blood from the abdominal aorta. Erythrocytes were seperated from heparinized whole blood samples. Deformability measurements were made in erythrocyte suspensions in phosphate buffered saline. A constant flow filtrometer system was used to measure erythrocyte deformability and relative resistance was calculated.
Results: It was found that the formation of ischemia reperfusion increases the relative resistance according to the control group (p < 0.0001). It was determined that application of pregabalin 50 or 200 mg did not change erythrocyte deformability in ischemia reperfusion-induced rats (p = 0.632, p = 0.811).
Conclusion: The administration of 50 or 200 mg of pregabalin has no negative effect on the erythrocyte deformability in ischemia reperfusion-induced rats. We think that pregabalin can be safely used for analgesia in the cases of IR. However, these findings should be supported by clinical and experimental studies carried out in more detailed and broader series.
Key words: Ischemia; Ischemia reperfusion; Ischemia Reperfusion Injury; Pregabalin; Dose; Erythrocyte deformability; Pregabalin; Gamma-Aminobutyric Acid
Citation: Özer A, Çomu FM, Demirtaş H, Kılıç Y, Mardin B, Öztürk L, İriz E, Arslan M, Küçük A. Effect of different doses of pregabalin on erythrocyte deformability in rats with lower limb ischemia reperfusion injury. Anaesth Pain & Intensive Care 2017;21(3):350-353
Received – 02 Feb 2017; Reviewed – 7 Feb & 21 Jul 2017; Corrected – 8 Feb, 31 Mar, 20 Jul 20717; Accepted – 12 Aug 2017
INTRODUCTION
Cellular damage after reperfusion of previously viable ischaemic tissues in lower extremity is a common and critical clinical incident. Reperfusion of an acutely ischemic tissue may, paradoxically, lead to systemic complications that are
associated with significant morbidity and mortality rates. As reperfusion progresses generally systemic inflammatory response syndrome and multiple organ failure (kidney, respiratory and circulatory system, etc.) follow local edema and muscle necrosis.1-5
Pregabalin, a GABA analogue, is a potent new generation antiepileptic drug that is used for the treatment of partial onset seizures, painful diabetic neuropathy, and post-herpetic neuralgia.6 It binds to the α2δ subunit of the voltage-dependent calcium channel and does not bind to the protein. Pregabalin does not interact with liver enzymes and 95% of it is excreted by kidneys. Besides these clinical indications, it has some new properties that we need to focus on. Pregabalin has been used to protect against IR injury in many organs.1,7,8
Benefits of pregabalin use to prevent local and distal tissue injury due to IR has been well documented so far. However, not much is known about its protective effect on erythrocyte deformability following IR injury. The primary aim of this study was to investigate deformability changes and the role of pregabalin in preventing these changes in the erythrocytes of rats in an experimental model of lower limb muscle IR injury.
METHODOLOGY
Animals and Experimental Protocol
This study was carried out in Gazi University Physiology Laboratory with the approval of the Ethics Committee of Experimental Animals of our university. All of the procedures were performed according to accepted standards of Guide for the Care and Use of Laboratory Animals.
In our study, 24 Wistar Albino rats weighing between 250 and 300 g, raised under the same environmental conditions, were used. The rats were kept under 20-21 oC at cycles of 12-hour daylight and 12-hour darkness and had free access to food until 2 hours before the anesthesia procedure. The animals were randomly separated into four groups, each containing 6 rats. Midline laparatomy was done under general anesthesia.
Control group: A midline laparotomy was performed without any extra surgical intervention. After 2 hours of follow-up blood sample was collected and then animals were sacrificed.
I/R group: Midline laparotomy was performed in the same way. Infrarenal aorta was clamped for 2 hours. The clamp was removed and then reperfusion was started. Reperfusion lasted for two more hours. In the end after blood sampling from their abdominal aorta rats were sacrificed.
I/R group with pregabalin 50 mg: Similiar steps were followed as mentioned above but in addition to the procedure pregabalin was given (50 mg/kg) intraperitoneally for 30 minutes before ischemia period. After collecting blood samples rats were sacrificed at the end of 2 hours reperfusion period.
I/R group with pregabalin 200 mg: Similiar steps were followed as mentioned above but in addition to the procedure pregabalin was given (200 mg/kg) intraperitoneally for 30 minutes before ischemia period. After collecting blood samples rats were sacrificed at the end of 2 hours reperfusion period.
Rats were anesthetized with ketamine (100 mg/kg, intraperitoneally) and intracardiac blood samples were collected. Erythrocyte packs were prepared using heparinized total blood samples. Erythrocytes packs were mixed with phosphate buffered saline (PBS) buffer to generate a suspension with the value of 5% HCT. Those erythrocyte suspensions were used for the measurement of deformability.
Deformability Measurements:
To prevent hemolysis samples of blood were collected very carefully and measurement process was done rapidly. Collected blood was centrifuged at 1000 rpm for ten minutes. Blood plasma in the upper phase and the buffy coat, which is a thin layer of leukocytes mixed with platelets in the middle on erythrocytes were removed. Isotonic PBS buffer was added to collapsing erythrocytes and this mixture was centrifuged at 1000 rpm for ten minutes. Liquid on the upper surface was taken. Washing process was repeated three times and finally pure red cell packs were obtained. Erythrocyte suspensions with 5% hematocrit in phosphate buffered saline (PBS) buffer were used to do deformability measurements. Erytrocytes were collected and then deformability measurements were done at 22 ºC.
Erytrocytes deformability was measured by the constant-current filtrometre system . Samples were prepared as 10 ml of erytrocytes suspension and PBS buffer before measurement. The infusion pump was set at 1.5 ml/min for a constant rate of flow. A 28 mm nucleoporin polycabonate filter with a 5 µm pore diameter was used Consisting pressure changes while the erythrocytes passing through from the filter were detected by the pressure transducer and the data was transferred to computer with the help of MP 30 data equation systems (Biopac Systems Inc, Commat, USA). At different times pressure changes were measured by using relevant computer programs for calculations. Pressure calibration of the system was performed each time before measuring the samples. After buffer (PT) was passed through the filtration system the erythrocytes (PE) were passed next. Pressure variations were measured. By relating the pressure value of erythrocytes suspension to pressure value of buffer, the relative refractory period value (Rrel) was calculated. The deformability index was interpreted; as Rrel increased the ability of erythrocyte deformability was affected adversely.
Statistical Analysis: All the data were processed by variance analysis in SPSS 17.0 for Windows statistical software. A p-value less than 0.05 was considered statistically important. The data were expressed as mean ± standard deviation. Variance analysis and Kruskal-Wallis test were used to evaluate the data. Mann Whitney U test with Bonferroni correction were used to evaluate the variables with significance.
RESULTS
The study showed that compared to control and IR groups, relative resistance – a marker of erythrocyte deformability, was increased significantly by IR (p < 0.0001) (Figure).|
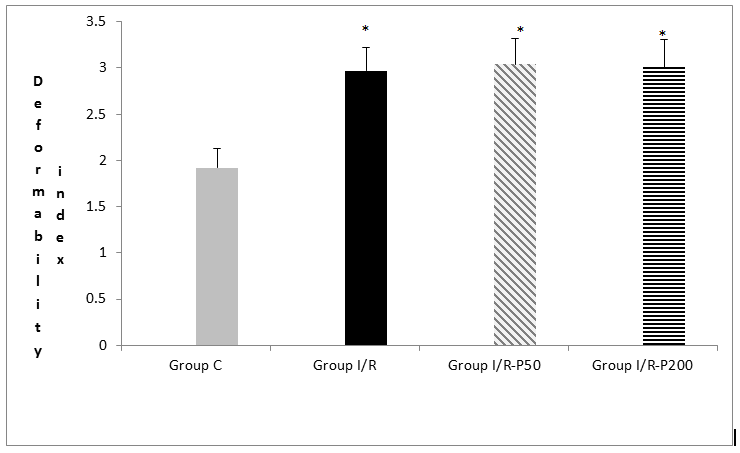
Figure: Erythrocyte deformability index values of the groups. Each bar represents the mean ± SD
* p < 0.05 compared to the Group C
The values of the control group were significantly lower than those of the IR, IR-P 50 and IR-P 200 mg groups (p < 0.0001, p < 0.0001, p < 0.0001, respectively). It was determined that application of pregabalin 50 or 200 mg did not change erythrocyte deformability in IR-induced rats (p = 0.632, p = 0.811, respectively).
DISCUSSION
Pregabalin is a structural analog of gamma-aminobutyric acid and has analgesic, anticonvulsant, anxiolytic and opioid-sparing effects. Pregabalin is a gabapentin derivative and has superior pharmacokinetic activity although it shows similar effect.8 In animal studies, pregabalin, such as gabapentin, has also been shown to be effective in several models of neuropathic pain, incisional and inflammatory injury.9 There are studies showing that preoperative administration of pregabalin reduces acute postoperative pain and analgesic consumption and the incidence of chronic neuropathic pain.10,11
Pregabalin is a gamma-amino-butyric acid analog with anticonvulsant, anxiolytic and opioid-sparing properties. In a study of epilepsy patients, oxcarbazepine has been shown to increase erythrocyte deformability at a shear force of 0.95 Pa.12
For being capable for carrying oxygen and vital molecules to the final organ capillaries and excretion of metabolic wastes, erythrocytes must be able to extend and curve to move in these areas. This capacity, called as “deformability” is more important in microcirculation. Altered erythrocyte deformability changes both oxygen delivery capacity of the erythrocytes and the survival of the circulating erythrocytes .13-15 In addition to these data our findings indicate that in rats undergoing IR erythrocyte deformability impairs and this impairment leads to disturbed microvascular perfusion and related problems.
CONCLUSION
So we think that erythrocyte deformability measurement can be a useful parameter in ischemia reperfusion injury. We also did not observe negative effect of pregabalin on maintaining erythrocyte deformability during periods of ischemia reperfusion but we still think these promising results should further be supported by more detailed studies with larger sample size.
Conflict of interest: Nil declared by the authors
Author contribution: AÖ: Main author to write the article, Concept, conduction of the study work and manuscript editing
FMÇ, LO: Wrote the manuscript
HD: Collection of data
YK, MA: Helped in experimental study and write the manuscript
BM: Helped in experimental study and collection of data
Eİ: help us to experimental study
AK: help us to results evaluation and write the article
REFERENCES
- Millecamps M, Coderre TJ. Rats with chronic post-ischemia pain exhibit an analgesic sensitivity profile similar to human patients with complex regional pain syndrome–type I. Eur J Pharmacol. 2008 Mar 31;583(1):97-102. doi: 10.1016/j.ejphar.2008.01.006. Epub 2008 Jan 26.[PubMed] [Free full text]
- Duru S, Koca U, Oztekin S, Olguner C, Kar A, Coker C, et al. Antithrombin III pretreatment reduces neutrophil recruitment into the lung and skeletal muscle tissues in the rat model of biletaral lower limb and reperfusion: A pilot study. Acta Anaesthesiol Scand. 2005 Sep;49(8):1142–8. [PubMed]
- Turchanyi B, Toth B, Racz I, Vandegh Z, Furesz J, Hamar J. Ischemia reperfusion injury of skeletal muscle after selective deafferantation. Physiol Res. 2005;54(1):25–32. [PubMed] [Free full text]
- Erer D, Özer A, Demirtaş H, Gönül II, Kara H, Arpacı H, et al. Effects of alprostadil and iloprost on renal, lung and skeletal muscle injury following hind limb ischemia-reperfusion injury in rats. Drug Des Devel Ther. 2016 Aug 19;10:2651-2658. doi: 10.2147/DDDT.S110529. eCollection 2016 [PubMed] [Free full text]
- Erer D, Dursun AD, Oktar GL, Iriz E, Zor MH, Elmas C, et al. The effects of iloprost on lung injury induced by skeletal muscle ischemia-reperfusion. Bratisl Lek Listy. 2014;115(7):405-410. [PubMed]
- Blommel ML, Blommel AL. Pregabalin: An antiepileptic agent useful for neuropathic pain. Am J Health Syst Pharm. 2007 Jul 15;64(14):1475-82. [PubMed]
- Kazanci B, Ozdogan S, Kahveci R, Gokce EC, Yigitkanli K, Gokce A, et al. Neuroprotective Effects of Pregabalin Against Spinal Cord Ischemia-Reperfusion Injury in Rats. Turk Neurosurg. 2016 Apr 27. doi: 10.5137/1019-5149.JTN.17959-16.1. [Epub ahead of print]. [PubMed]
- Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45 Suppl 6:13-8. [PubMed] [Free full text]
- Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007 Dec;105(6):1805-15. [PubMed]
- Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011 Apr;106(4):454-62. doi: 10.1093/bja/aer027. Epub 2011 Feb 26. [PubMed] [Free full text]
- Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010 Jan 1;110(1):199-207. doi: 10.1213/ANE.0b013e3181c4273a. Epub 2009 Nov 12[PubMed]
- Genç O, Erken G, Erken HA, Bolukbasi N, Bolukbasi O, Kucukatay MB. Parsiyel Epilepsili Hastalarda Antiepileptik Monoterapisinin Hemoreolojik parametreler Üzerindeki Etkisi. Türk Fizyolojik Bilimler Derneği 36. Ulusal Fizyoloji Kongresi P18, 14-17 Eylül 2010 Edirne,2010.
- Zinchuk VV. Erythrocyte deformability: physiological aspects. Usp Fiziol Nauk. 2001 Jul-Sep;32(3):66-78. [PubMed]
- Kuypers FA. Red cell membrane damage. J Heart Valve Dis. 1998 Jul;7(4):387-95. [PubMed]
- Sivilotti ML. Oxidant stres and haemolysis of the human erythrocyte. Toxicol Rev. 2004;23(3):169-88. [PubMed]

