Beena Thada, MD1, Arvind Khare, MD2, Surendra Kumar Sethi,MD1, Shriphal Meena,MD3, Manish Verma, MD4
1Assistant Professor; 2Associate Professor
Department of Anesthesiology, JLN Medical College & Hospital, Ajmer, Rajasthan, (India)
3Professor, Department of Anesthesiology, SMS Medical College & Attached Hospitals,
Jaipur, Rajasthan, (India)
4Junior Specialist (Pediatrics), Satellite Hospital, Ajmer, Rajasthan, (India)
Correspondence: Dr Surendra Kumar Sethi, MD, Assistant Professor, Department of Anesthesiology, JLN Medical College and Hospital, Ajmer, Rajasthan, India; Phone: +919587150598; E-mail: drsurendrasethi80@gmail.com
ABSTRACT:
Background: Subarachnoid block is still the most commonly used anesthetic technique for lower abdominal surgeries, however local anesthetics alone are associated with relatively short duration of action. The intrathecal adjuvants has been reported to improve the quality of anesthesia along with prolongation of postoperative analgesia and has gained popularity nowadays. So the aim of our study was to compare the dexmedetomidine and fentanyl as intrathecal adjuvant to 0.5% hyberbaric bupivacaine with respect to onset and duration of sensory and motor block, duration of analgesia, hemodynamic variations and incidence of side effects.
Methodology: Sixty four female patients, aged 30-60 years, belonging to American Society of Anesthesiologists (ASA) physical status І or ІІ, scheduled for elective total abdominal hysterectomy with or without bilateral salpingo-oophorectomy were randomly allocated into two groups, Group BD received 2.5 ml of 0.5% hyperbaric bupivacaine and 5 μg dexmedetomidine diluted in 0.5 ml preservative free normal saline while Group BF received 2.5 ml of 0.5% hyperbaric bupivacaine and 25 μg (0.5 ml) fentanyl.
Results: There was no statistically significant difference between two groups with respect to onset of sensory and motor block (p > 0.05). The mean time for two segment sensory regression was significantly slower in Group BD as compared to Group BF (p < 0.05). Patients in Group BD had significantly prolonged duration of sensory and motor block as compared to Group BF (p < 0.05). Similarly the duration of analgesia was significantly prolonged in Group BD (p < 0.05), along with reduced requirement of rescue analgesics. The patients in both groups did not show any significant difference with respect to hemodynamic changes and incidence of side effects (p > 0.05).
Conclusion: Dexmedetomidine as intrathecal adjuvant was found to have prolonged sensory and motor block, provide good quality of intraoperative analgesia, stable hemodynamics, minimal side effects and prolonged postoperative analgesia along with reduced demand for rescue analgesics as compared to fentanyl.
Key words: Subarachnoid block; Intrathecal adjuvant; Dexmedetomidine; Fentanyl; Bupivacaine; Total abdominal hysterectomy
Citation: Thada B, Khare A, Sethi SK, Meena S, Verma M. Comparison of dexmedetomidine and fentanyl as intrathecal adjuvants to 0.5% hyperbaric bupivacaine for total abdominal hysterectomy under subarachnoid block: A prospective randomized double blind study. Anaesth, Pain & Intensive Care 2017;21(1):65-72
Received: 29 Dec 2016; Reviewed: 27 Feb, 20 Mar 2017; Accepted: 30 Mar 2017
INTRODUCTION:
Among all the regional anesthetic techniques used for lower abdominal surgeries, subarachnoid block is still the most commonly used technique as it is easy to perform, has rapid onset of anesthesia, provides adequate muscle relaxation with excellent operating conditions, more economical and has less failure rates.1,2 But the major disadvantage with subarachnoid block using local anesthetics alone, is its relatively short duration of action and inadequate postoperative analgesia.2
Various intrathecal adjuvants like fentanyl, morphine, dexmedetomidine, clonidine, neostigmine, and ketamine are being increasingly used with local anesthetics nowadays. These adjuvants prolong the duration of block associated with improved quality of block, reduces the local anesthetic dose requirement along with their side effects simultaneously achieving better patient satisfaction and faster recovery.3
Fentanyl is a potent, short acting, lipophilic synthetic opioid analgesic commonly used as an adjuvant for postoperative analgesia however it might be associated with some adverse effects like sedation, hypotension, respiratory depression, pruritus and nausea or vomiting so there is constant need to search a drug which provides adequate intraoperative as well as postoperative analgesia, along with prolonged duration of block and minimal side effects.4,5 Dexmedetomidine ─ a highly selective alpha-2 adrenergic agonist is emerging as a useful intrathecal adjuvant and gained popularity as it has been reported to potentiate the effect of local anesthetics and prolongs both the duration of block and postoperative analgesia along with stable hemodynamics and minimal side effects.6-9
So based on the above hypothesis, this prospective randomized double blind study was aimed to compare the efficacy of dexmedetomidine with fentanyl given as an intrathecal adjuvant along with bupivacaine for onset and duration of sensory and motor blockade, duration of analgesia, hemodynamic variations and incidence of side effects.
METHODOLOGY:
After obtaining approval from the institutional ethical committee and written informed consent, this prospective, randomized, double blind study was conducted including sixty four patients of female gender, aged 30-60 years, weighing 45-70 kg, belonging to ASA (American Society of Anesthesiologists) physical status I or II undergoing elective total abdominal hysterectomy with or without bilateral salpingo-oophorectomy under subarachnoid block. Patients with any deformity or local sepsis in spinal lumbar region, severe hypovolemia, increased intracranial pressure, major pre-existing neurological, cardiovascular, metabolic, hepatic, respiratory or renal disease; bleeding or coagulation abnormalities, history of allergy or hypersensitivity to drugs, patients with anemia (Hb < 10 gm%), patients on therapy with adrenergic receptor antagonists, calcium channel blockers, and, or ACE inhibitors were excluded from the study.
After arrival in the operation theatre, two intravenous (IV) lines were taken with 20 G cannula and all patients were hydrated with 500 to 1000 ml ringer lactate solution preoperatively. All the standard monitors including pulse oximeter (SpO2), non-invasive blood pressure (NIBP) and electrocardiogram (ECG) were attached to the patient and baseline parameters were recorded. All patients received premedication as ranitidine 50 mg, metoclopramide 10 mg and midazolam 1 mg IV. Under full aseptic precautions and with the patient in the left lateral position subarachnoid block was performed at the L3–L4 intervertebral space using 25 G Quinke spinal needle and 3 ml of drug was injected over 30 sec.
Group BD received 2.5 ml of 0.5% hyperbaric bupivacaine and 5 μg dexmedetomidine diluted in 0.5 ml preservative free normal saline [normal saline was added to 1 ml (100 μg/ml) of dexmedetomidine to make it 10 ml (10 μg/ml) from this, 0.5 ml (5μg) of solution was taken with the help of 1 ml tuberculin syringe] while Group BF received 2.5 ml of 0.5% hyperbaric bupivacaine and 25 μg (0.5 ml) fentanyl. Patients were laid in supine position with 15° head down tilt immediately after subarachnoid block to achieve level of block of T5-T6 as required for surgery. Randomization was done by simple chit in box method. The drug combinations used in our study were prepared by one anesthesiologist and given by another anesthesiologist who were not involved in the study for the purpose of double blinding. Oxygen was given to all patients by venti mask @ 3 L/min.
The time at intrathecal injection was considered as zero (0) and following parameters were noted as soon as patients were supine; heart rate (HR), NIBP, and SpO2; time of onset of sensory block and highest level achieved by pin prick bilaterally at mid-clavicular line, time of onset of motor block by using modified Bromage scale,10 duration of surgery, side effects like hypotension, bradycardia, respiratory depression (defined as arterial oxygen saturation less than 90%), shivering, and nausea or vomiting.
Ephedrine 5 mg IV was given to treat intra-operative hypotension (defined as mean arterial blood pressure MAP < 70 mmHg), and atropine 0.3-0.5 mg IV was given to treat bradycardia (defined as heart rate < 50 bpm). Intraoperative nausea or vomiting was treated with ondansetron 2-4 mg IV. NIBP, HR and SpO2 were recorded at 1, 5, 10, 15, 20, 30, 60 and 120 min, and post operatively at 30 min intervals until rescue analgesic was given.
The level of sensory block was tested at frequent intervals of time till the highest level of the block reached and then, postoperatively, at 2 hour intervals till the patient complained of pain. Onset of sensory block was defined as the time taken to achieve highest level of sensory block and the onset of motor block was defined as the time taken to achieve Bromage grade 3 block (complete motor block) from the time of subarachnoid block.
Immediately after operation patients were shifted to recovery room and HR, NIBP and SpO2 were recorded at regular intervals of 30 min for four hours. Two segment regression time was taken as time of regression of sensory block by two segments from the highest level attained.
Duration of sensory block was measured as the time taken for the sensory block to regress up to S1 dermatome (i.e. the heel) from the highest level achieved.
Postoperatively, the pain scores will be recorded by using visual analog pain scale11 (VAS 0 to 10), initially every hours for 2 h, then every 2 h for the next 8 h and then after every 4 h till 24 h. Visual analogue score read; 0: no pain; 1-3: mild pain; 4-6: moderate pain; 7-9: severe pain; and 10: the worst imaginable pain. Patient’s first demand for rescue analgesia constituted the end point of the study. Patients were allowed to receive rescue analgesics (intramuscular diclofenac) on demand or on VAS > 4.
Duration of analgesia was measured as time from the drug given in subarachnoid space to the patient’s first request for rescue analgesic. Modified Bromage score was used to assess duration of motor block [0 = able to move the hip, knee, and ankle; 1 = unable to move the hip but is able to move the knee and ankle; 2 = unable to move the hip and the knee but able to move the ankle; 3 = unable to move the hip, knee, or ankle].
Duration of motor block was measured by recording the time elapsed from maximum to the lowest Bromage score i.e. regression to Bromage 0. Patients were observed for a period of 24 hours for any side effects like sedation, nausea / vomiting, pruritus, urinary retention, bradycardia or hypotension. Ramsay Sedation Score12 was used to assess sedation postoperatively in patients.
Statistical analysis: Sample size was calculated at 80% study power and alpha level of 0.05 assuming standard deviation of time of sensory and motor block of 20 min and difference of mean to be detected of 10 min. The sampling error was kept at 10%. This sampling size obtained comes to be 32 patients in each group. Statistical analysis was performed with the SPSS version 15.0 for Windows statistical software package (SPSS Inc., Chicago, IL, USA). Categorical data i.e. ASA grade, type of surgery and the incidence of adverse effects are presented as numbers (percent) and compared among groups using Chi square test. p value < 0.05 was considered statistically significant. Groups are compared for demographic data (age, weight), duration of surgery, onset of motor block, sensory block, highest level achieved, time for two segment regression, VAS score, total duration of sensory block, motor block and analgesia by analysis of variance (ANOVA) and t-test. Data are represented as mean ± SD.
RESULTS:
The demographic profile was comparable between the two groups with respect to age, weight, type and duration of surgery (Table 1).
Table 1: Demographic data
| Variable | Group BD
(n = 32) |
Group BF
(n = 32) |
p-value |
| Age(y) | 41.5 ± 5.2 | 40.7 ± 5.2 | 0.5633 |
| Weight(kg) | 61.0 ± 4.2 | 58.9 ± 4.3 | 0.0587 |
| Duration of surgery(min) | 59.1 ± 9.2 | 58.8 ± 8.9 | 0.8905 |
| Type of surgery | |||
| TAH+BSO | 20 (62.5%) | 20 (62.5%) | – |
| TAH | 12 (37.5%) | 12 (37.5%) | – |
*Values are expressed as Mean ± SD and n (%)
*TAH – Total abdominal hysterectomy, BSO – bilateral salpingo-oophorectomy
P < 0.05 (significant)
There was no statistically significant difference between two groups with respect to onset of sensory block, (p > 0.05). The mean time for onset of sensory block was 10.9 ± 0.9 min and 10.9 ± 1.1 min in Groups BD and BF respectively. There was no statistically significant difference in the highest level of sensory block achieved in the two groups (T6.5 ± 0.9 in each group) or in the time to reach the highest level (p > 0.05)(Table 2).
The mean time for two segment sensory regression was 117.5 ± 9.7 min in Group BD and 76.1 ± 8.7 min in Group BF (p = 0.0000) which was highly significant. The mean duration of sensory block was 471.8 ± 8.9 min in Group BD and 179.6 ± 6.6 min in Group BF (p = 0.0000) which was also highly significant (Table 2). Both, the time to two segment regression and time to S1 regression were significantly prolonged in Group BD (p < 0.05).
There was no statistically significant difference between two groups with respect to onset of motor block (p > 0.05). The onset of motor block was 7.8 ± 1.0 min and 7.5 ± 1.0 min in Groups BD and BF respectively (p = 0.1266). The duration of motor block was 421.1 ± 10.5 min and 153.1 ± 6.3 min in Group BD and B-F respectively (p = 0.0000) which was found to be statistically significant (p < 0.05). So similarly the time to regression of motor block to Bromage 0 (no block) was significantly prolonged in dexmedetomidine group (Table 2).
The mean duration of analgesia in the postoperative period was 260.4 ± 13.0 min in Group BD and 161.8 ± 8.2 min in Group BF (p = 0.0000), and statistically significant difference (p < 0.05) was found when Group BD was compared with group BF. The time to rescue analgesic was significantly longer in Group BD as compared to Group BF. The requirement of diclofenac in the first 24 h was significantly lower in Group BD as compared to Group BF (Table 2).
Table 2: Characteristics of subarachnoid block (Data presented in minutes)
| Parameter | Group BD
(n = 32) |
Group BF
(n = 32) |
p-value |
| Onset of sensory block | 10.9 ± 0.9 | 10.9 ± 1.1 | 1.0000 |
| Highest sensory level | T6.5 ± 0.9 | T6.5 ± 0.9 | 0.7856 |
| Time for 2 segment regression | 117.5 ± 9.7 | 76.1 ± 8.7 | 0.0000 |
| Duration of sensory block | 471.8 ± 8.9 | 179.6 ± 6.6 | 0.0000 |
| Onset of motor block | 7.8 ± 1.0 | 7.5 ± 1.0 | 0.1266 |
| Duration of motor block | 421 ± 10.5 | 153.1 ± 6.3 | 0.0000 |
| Total duration of analgesia | 260.4 ± 13 | 161.8 ± 8.2 | 0.0000 |
Values are expressed as Mean ± SD; p < 0.05 or 0.01 (significant); p < 0.001 (highly significant).
The patients in both groups remained hemodynamically stable intraoperatively. There was significant difference in heart rate over time in both groups, but there was no significant difference among two groups in the pattern of decrease in heart rate (p > 0.05) (Figure 1). Similarly there was significant difference in mean arterial pressure over time in both groups, but there was no significant difference among two groups in the pattern of decrease in mean blood pressure (p > 0.05) (Figure 2).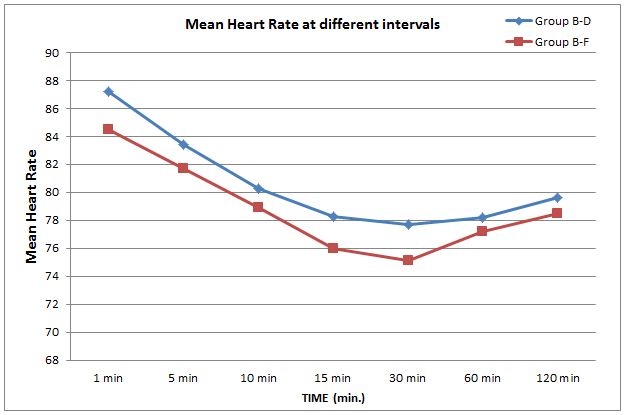
Figure 1: Comparison of mean heart rate at various time intervals
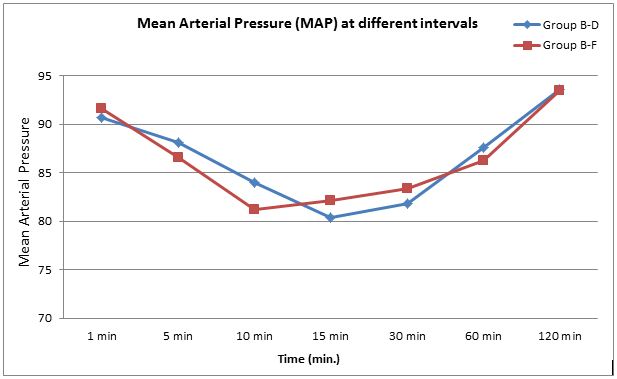
Figure 2: Comparison of mean arterial pressure at various time intervals
In our study however, hypotension was more in the Group BD than in Group BF, but it was statistically insignificant. One patient in Group BD had bradycardia (HR < 50/min) but it was managed successfully with atropine 0.5 mg IV. Patients in both groups did not show statistically significant difference in the incidence of adverse effects (Table 3).
Table 3: Characteristics of hemodynamics and incidence of side effects (intraoperative and early postoperative period)
| Side effects | Group BD
(n = 32) |
Group BF
(n = 32) |
p-value |
| Hypotension | 4 (12.5) | 3 (9.37) | > 0.05 |
| Bradycardia | 1 (3.12) | 0 (0) | > 0.05 |
| Nausea, Vomiting | 1 (3.12) | 2 (6.25) | > 0.05 |
| Pruritus | 0 (0) | 1 (3.12) | > 0.05 |
| Urinary retention | 0 (0) | 1 (3.12) | > 0.05 |
Values are expressed as number (percentage); p > 0.05 (not significant)
None of the patients experienced respiratory depression or arterial oxygen desaturation. Two patients in fentanyl group (3.12%) and one patient of dexmedetomidine group (6.25%) experienced nausea and vomiting which was not statistically significant (p > 0.05). One patient of fentanyl group experienced pruritus and urinary retention while none from dexmedetomidine group (p > 0.05) (Table 3). The sedation score was significantly more in Group BD patients. The mean sedation score was 1.5 ± 0.5 in Group BD as compared to 1.1 ± 0.2 in Group BF, which was statistically significant (p < 0.05).
DISCUSSION:
Dexmedetomidine, a new highly selective α-2 agonist, is emerging as an intrathecal adjuvant with local anesthetics as it provides adequate intraoperative analgesia along with prolonged postoperative analgesia, stable hemodynamics and minimal side effects. The affinity of dexmedetomidine to α-2 adrenoceptor agonists is 10 times as compared to clonidine, reported by Kalso et al.8
The mechanism by which intrathecal α-2 adrenergic agonists prolong sensory and motor block is not clear. However, dexmedetomidine acts by binding to presynaptic C-fibers and post-synaptic dorsal horn neurons and their analgesic action is due to inhibition of the release of C-fiber transmitters and hyperpolarization of postsynaptic dorsal horn neurons. Intrathecal dexmedetomidine has been found to have antinociceptive action for both somatic as well as visceral pain.2,6,13
Local anesthetics act by blocking sodium channels and the synergistic effect of local anesthetic and α-2 adrenoceptor agonist seems to prolong the duration of action of local anesthetics given intrathecally, while the prolongation of motor block may result from the binding of α-2 adrenoceptor agonists (dexmedetomidine) to motor neurons in the dorsal horn.6,14 Various studies were conducted using intrathecal dexmedetomidine along with bupivacaine in human beings but no postoperative neurological deficit has been reported yet.1,9
Dexmedetomidine causes dose dependent decreases in heart rate and blood pressure associated with concomitant decrease in the level of plasma catecholamines which would be of considerable benefit in patients with tachycardia and hypertension, and dexmedetomidine typically improves hemodynamic stability in the perioperative period. Intrathecal local anesthetics decrease mean arterial pressure and sympathetic outflow, presumably by blocking axonal transmission along spinal roots and nerves.3
Fentanyl acts through combining with opioid receptors in the dorsal horn of spinal cord and may also have its action via supraspinal spread when given intrathecally and has been used as an adjuvant to local anesthetics in subarachnoid block, and reduces both visceral and somatic pain but its use is now limited due to dose dependent adverse effects associated with it.15,16
Our study compared dexmedetomidine (5 µg) with fentanyl (25 µg) used as an intrathecal adjuvant to local anesthetic (bupivacaine 0.5% heavy) and evaluated the analgesic efficacy of intrathecal dexmedetomidine in terms of different parameters and to find an adequate dose of dexmedetomidine for using as an intrathecal adjuvant. The results of our study showed that addition of 5 µg dexmedetomidine significantly prolonged both the sensory and motor block along with superior quality of block compared with 25 µg fentanyl given intrathecally with hyperbaric bupivacaine.
The time of onset of sensory block was comparable between the two groups. Our results coincide with the findings of Gupta et al17 , Mahendru et al6 and Al Ghanem et al.18 Although they have used similar doses of dexmedetomidine (5 µg) and fentanyl (25 µg) in their study, the onset times of sensory block observed in the studies done by Al Ghanem et al. and Mahendru et al. were relatively shorter as compared to our study and this was attributed due to difference in criteria to achieve the level of block as the criteria for the onset of block in our study was the time taken to achieve the highest level (T6) of sensory block whereas it was the time taken to achieve T8 level in studies done by Mahendru et al. and Al Ghanem et al. Similarly there was no significant difference between the two groups with regard to onset of motor block which were consistent with the findings of Mahendru et al.6, Al Ghanem et al.18 and Al Mustafa et al.15 The difference in results obtained by different authors regarding onset of motor block may be due to different volumes, concentration and baricity of local anesthetic solutions used. In our study the time for 2 segment regression was significantly prolonged in dexmedetomidine group as compared to fentanyl group, which showed the improved quality of block in dexmedetomidine group. Similar results were also observed by above authors as well.19
The duration of sensory block was 471.8 ± 8.9 min and 179.6 ± 6.6 min in Groups BD and BF respectively which was statistically significant, (p < 0.05). Similarly the duration of motor block was 421.1 ± 10.5 min and 153.1 ± 6.3 min in Group BD and BF respectively, which was statistically significant between the two groups (p < 0.05). Our study has shown that dexmedetomidine (5 µg) as an adjuvant with hyperbaric bupivacaine significantly prolongs both sensory and motor block compared with fentanyl (25 µg) given intrathecally.
Our results coincide with Al-Ghanem et al. who had studied the effect of addition of 5 μg dexmedetomidine or 25 μg fentanyl intrathecally to 10 mg isobaric bupivacaine in vaginal hysterectomy and concluded that 5 μg dexmedetomidine produced more prolonged motor and sensory block as compared with 25 μg fentanyl. Similarly Mahendru et al., Kanazi et al.9 Al Mustafa et al.15 and Hala E et al.20 found significantly prolonged durations of sensory and motor block and observed dose dependent prolongation of sensory and motor blockade with increasing dose of dexmedetomidine. The prolongation of sensory block may be attributed to synergism between local anesthetics and dexmedetomidine whereas prolongation of motor block may result from the binding of dexmedetomidine to motor neurons in dorsal horn.2
In our study, the mean duration of analgesia was 260.4 ± 13.0 min and 161.8 ± 8.2 min in Group BD and BF respectively which was statistically significant (p < 0.05).The total duration of analgesia was significantly prolonged in dexmedetomidine group. Diclofenac 75 mg was given intramuscularly as rescue analgesic. In our study, Group BD required 150 mg diclofenac whereas Group BF required 225 mg diclofenac in 24 h given as rescue analgesic. Our study has shown that the addition of 5 μg dexmedetomidine with 0.5% hyperbaric bupivacaine significantly prolongs the duration of analgesia as compared to Group BF and reduced the rescue analgesic requirement significantly. Our results coincides with findings of other authors.6,15,17,20,21 Al Mustafa et al. reported the reduced analgesic requirement in the dose dependent pattern when comparing with higher doses of dexmedetomidine (10 µg).
No clinically significant difference in the hemodynamic parameters and adverse effects were reported between the two groups. In our study, however, hypotension and bradycardia were more in the Group BD than in the fentanyl group, but it was statistically insignificant. Similarly pruritus after intrathecal fentanyl is well known but it was found to be insignificant in our study. Talke et al. observed the antishivering properties of α-2 adrenergic agents.22 We did not find any incidence of shivering in the two groups. Nausea and vomiting were observed in 3.12% and 6.25% patients in Group BD and BF respectively. This suggested that the incidence of nausea and vomiting was not significantly different among the groups. Similar results were found in earlier studies.6,9,18,23,24
Although the patients in both groups remained hemodynamically stable intraoperatively, the mean sedation score was significantly more in patients in Group BD. It was 1.5 ± 0.5 in Group BD as compared to 1.1 ± 0.2 in Group BF which was statistically significant (p < 0.05), however this was in acceptable range as we have used lower dose of dexmedetomidine and patients remained easily arousable and co-operative.
CONCLUSION:
Intrathecal 5 µg dexmedetomidine proved to be a better alternative to 25 µg fentanyl as an adjuvant to 0.5% hyperbaric bupivacaine in subarachnoid block for lower abdominal surgeries as it was found to be associated with prolonged motor and sensory blockade, provides good quality of intraoperative analgesia, stable hemodynamics, minimal side effects and prolonged postoperative analgesia along with reduced demand for rescue analgesics as compared to fentanyl. Although higher doses (10-15 µg) of intrathecal dexmedetomidine as an adjuvant might provide more prolonged sensory and motor block along with prolonged duration of analgesia but at the cost of increased side effects, more hemodynamic variations and more sedation which is very much undesirable and hence 5 µg seems to be adequate dose to be used as intrathecal adjuvant.
Acknowledgement: Nil
Funding: None
Conflict of interest: None
Author contribution: BT: Literature search and review, concepts, conduction of study work, manuscript preparation
AK: Concepts and design, manuscript editing and review
SKS: Literature review, manuscript editing and review
SM: Concepts and design, literature review
MV: Manuscript editing
REFERENCES:
- Abdelhamid SA, El-lakany MH. Intrathecal dexmedetomidine:Useful or not? J Anesth Clin Res. 2013;4(9):351. [Free full text]
- El-Attar A, Aleem MA, Beltagy R, Ahmed W. A comparative study of intrathecal dexmedetomidine and fentanyl as additives to bupivacaine. Res Opin in Anesth Intensive Care 2015;1:43-49. [Free full text]
- Shukla D, Verma A, Agarwal A, Pandey HD, Tyagi C. Comparative study of intrathecal dexmedetomidine with intrathecal magnesium sulphate used as adjuvants to bupivacaine.J Anaesthesiol Clin Pharmacol. 2011 Oct;27(4):495-499. doi: 10.4103/0970-9185.86594. [PubMed] [Free full text]
- Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanyl and sufentanyl. Anesthesiology. 2000 Mar;92(3):739-753. [PubMed] [Free full text]
- Hamber EA, Viscomi CM. Intrathecal lipophilic opioids as adjuncts to surgical spinal anesthesia. Reg Anesth Pain Med. 1999 May;24(3):255-63. [PubMed]
- Mahendru V, Tewari A, Katyal S, Grewal A, Singh MR, Katyal R. A comparison of intrathecal dexmedetomidine, clonidine, and fentanyl as adjuvants to hyperbaric bupivacaine for lower limb surgery: a double blind controlled study. J Anaesthesiol Clin Pharmacol. 2013 Oct;29(4):496-502. [PubMed] [Free full text]
- Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth. 2001 Nov;87(5):684-90. [PubMed]
- Kalso E, Poyhia R, Rosemberg P. Spinal antinociceptive by dexmedetomidine, a highly selective alpha 2-adrenergic agonist. Pharmacol Toxicol. 1991 Feb;68(2):140-3.[PubMed]
- Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anesthesiol Scand. 2006 Feb;50(2):222-7. [PubMed] [Free full text]
- Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand Suppl. 1965;16:55-69.[PubMed]
- Katz J, Melzack R. Measurement of pain. Surg Clin North Am. 1999 Apr;79(2):231-52. [PubMed]
- Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled sedation with alpaxalone-alphadolone. Br Med J.1974 Jun 22;2(5920):656-659. [PubMed] [Free full text]
- Eisenach JC, De Kock M, Klimscha W. Alpha2-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995). Anesthesiology. 1996 Sep;85(3):655-74. [PubMed] [Free full text]
- Harada Y, Nishioka K, Kitahata LM, Kishikawa K, Collins JG. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50, 488H. Anesthesiology. 1995 Aug;83(2):344-352. [PubMed] [Free full text]
- Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM,et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J. 2009 Mar;30(3):365-70. [PubMed]
- Benhamou D, Thorin D, Brichant JF, Dailland P, Milon D, Schneider M. Intrathecal clonidine and fentanyl with hyperbaric bupivacaine improves analgesia during cesarean section. Anesth Analg. 1998 Sep;87(3):609–13. [PubMed]
- Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A Comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to Bupivacaine. J Anaesthesiol Clin Pharmacol. 2011 Jul;27(3):339-43. doi: 10.4103/0970-9185.83678[PubMed] [Free full text]
- Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, Abu-Ali HM. Effect of Adding Dexmedetomidine versus Fentanyl to Intrathecal Bupivacaine on Spinal Block Characteristics in Gynecological Procedures: A Double Blind Controlled Study. Am J Appl Sci. 2009;6:882–7.
- Ibrahim FA. A comparative study of adding intrathecal dexmedetomidine versus sufentanil to heavy bupivacaine for postoperative analgesia in patients undergoing inguinal hernia repair. Benha M.J 2009;26:207-17.
- Hala EA, Shafie MA, yousef H. Dose –related prolongation of hyperbaric bupivacaine spinal anesthesia by dexmedetomidine. Ain Shams J of Anesthesiol 2011; 4:83-95. [Free full text]
- Mohamed AA, Farees KM, Mohamed SA. Efficacy of intrathecally administered dexmedetomidine versus dexmedetomidine with fentanyl in patients undergoing major abdominal cancer surgery. Pain Physician. 2012 Jul-Aug;15(4):339-348. [PubMed]
- Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997 Oct;87(4):835-841. [PubMed] [Free full text]
- Sunil BV, Sahana KS, Jajee PR. Comparison of dexmedetomidine, fentanyl and magnesium sulfate as adjuvants with hyperbaric bupivacaine for spinal anaesthesia: a double blind controlled study. Int J Recent Trends Sci Tech 2013;9:14-19. [Free full text]
- Safdari F, Aminnejad R, Mohajerani SA, Farivar F, Mottaghi K, Safdri H. Intrathecal dexmedetomidine and fentanyl as adjuvant to bupivacaine on duration of spinal block in addicted patients. Anesth Pain Med.2016 Jan 31;6(1):e26714. doi: 10.5812/aapm.26714. [PubMed] [Free full text]

